Details of the Drug Combinations
General Information of This Drug (ID: DMEYLH9)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ribavirin; 36791-04-5; Tribavirin; Rebetol; Virazole; Ribavirine; Copegus; Vilona; Ribamide; Ribasphere; Ribamidil; Viramid; Ribamidyl; Ribavirinum; Ribavirina; Rebetron; Varazid; RTCA; Ribavirin Capsules; Ribavirinum [INN-Latin]; Ribavirine [INN-French]; Ribavirina [INN-Spanish]; ICN-1229; Rebretron; Virazid; Ribav; 1-beta-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide; DRG-0028; 1-beta-D-Ribofuranosyl-1H-1,2,4-triazole-3-carboxamide; UNII-49717AWG6K; Ribavirin (Copegus); Copegus; Cotronak; RBV; RTC; Ravanex; Ribacine; Ribovirin; Viramide; Virazide; R 9644; SCH 18908; C-Virin; Copegus (TN); Drug: Ribavirin; KS-1104; R-964; RG-964; Rebetol (TN); Ribasphere (TN); Ribavirin [USAN:INN]; Vilona (TN); Virazole (Ribavirin) Inhalation Solution; Virazole (TN); AA-504/07617051; Ro 20-9963/000; Ro-20-9963; Ribavirin (JAN/USP/INN); 1-beta-D-Ribofuranosyl-1,2,4-triazolo-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide; RBI034 (2-5A antisense compound) + Ribavirin
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
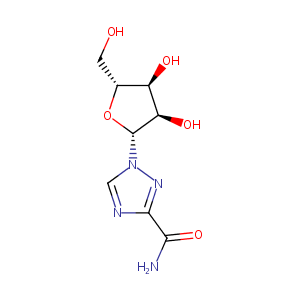 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
