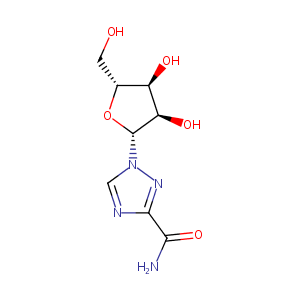
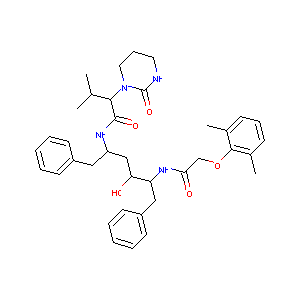
| 1 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Ribavirin FDA Label
|
| 4 |
Proteomics of SARS-CoV-2-infected Host Cells Reveals Therapy Targets. Nature. 2020 May 14. doi: 10.1038/s41586-020-2332-7.
|
| 5 |
SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016 Aug;14(8):523-34.
|
| 6 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 7 |
Glutathione peroxidase, thioredoxin, and membrane protein changes in erythrocytes predict ribavirin-induced anemia. Clin Pharmacol Ther. 2005 Oct;78(4):422-32. doi: 10.1016/j.clpt.2005.07.002.
|
| 8 |
Ribavirin inhibits the growth and ascites formation of hepatocellular carcinoma through downregulation of type I CARM1 and type II PRMT5. Toxicol Appl Pharmacol. 2022 Jan 15;435:115829. doi: 10.1016/j.taap.2021.115829. Epub 2021 Dec 14.
|
| 9 |
Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006 Jan;57(1):8-13.
|
| 10 |
Transport of levovirin prodrugs in the human intestinal Caco-2 cell line. J Pharm Sci. 2006 Jun;95(6):1318-25.
|
| 11 |
Identification and functional analysis of variants in the human concentrative nucleoside transporter 2, hCNT2 (SLC28A2) in Chinese, Malays and Indians. Pharmacogenet Genomics. 2007 Sep;17(9):783-6.
|
| 12 |
Kinetic study of anti-viral ribavirin uptake mediated by hCNT3 and hENT1 in Xenopus laevis oocytes. Biophys Chem. 2010 Mar;147(1-2):59-65.
|
| 13 |
Effects of dipyridamole coadministration on the pharmacokinetics of ribavirin in healthy volunteers. Drug Metab Pharmacokinet. 2013;28(5):406-10.
|
| 14 |
Functional analysis of purine nucleoside phosphorylase as a key enzyme in ribavirin metabolism. Drug Metab Pharmacokinet. 2014;29(2):211-4.
|
| 15 |
Phosphorylation of ribavirin and viramidine by adenosine kinase and cytosolic 5'-nucleotidase II: Implications for ribavirin metabolism in erythrocytes. Antimicrob Agents Chemother. 2005 Jun;49(6):2164-71.
|
| 16 |
Ribavirin inhibits colorectal cancer growth by downregulating PRMT5 expression and H3R8me2s and H4R3me2s accumulation. Toxicol Appl Pharmacol. 2021 Mar 15;415:115450. doi: 10.1016/j.taap.2021.115450. Epub 2021 Feb 9.
|
| 17 |
Ribavirin augments doxorubicin's efficacy in human hepatocellular carcinoma through inhibiting doxorubicin-induced eIF4E activation. J Biochem Mol Toxicol. 2018 Jan;32(1). doi: 10.1002/jbt.22007. Epub 2017 Nov 7.
|
| 18 |
Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999 Mar;30(3):376-82. doi: 10.1016/s0168-8278(99)80093-2.
|
| 19 |
Direct evidence for immunomodulatory properties of ribavirin on T-cell reactivity to hepatitis C virus. Antiviral Res. 2007 Jul;75(1):36-42. doi: 10.1016/j.antiviral.2006.11.008. Epub 2006 Dec 13.
|
| 20 |
Profiling the immunotoxicity of chemicals based on in vitro evaluation by a combination of the Multi-ImmunoTox assay and the IL-8 Luc assay. Arch Toxicol. 2018 Jun;92(6):2043-2054. doi: 10.1007/s00204-018-2199-7. Epub 2018 Mar 29.
|
| 21 |
Normal erythropoietin response in chronic hepatitis C patients with ribavirin-induced anaemia. Antivir Ther. 2003 Feb;8(1):57-63.
|
| 22 |
Inhibition of cardiomyocyte differentiation of human induced pluripotent stem cells by Ribavirin: Implication for its cardiac developmental toxicity. Toxicology. 2020 Apr 15;435:152422. doi: 10.1016/j.tox.2020.152422. Epub 2020 Feb 26.
|
| 23 |
Correlation of sICAM-1 and sVCAM-1 level with biochemical, histological and viral findings in chronic hepatitis C after interferon-alpha + ribavirin therapy. Rom J Gastroenterol. 2003 Jun;12(2):91-5.
|
| 24 |
Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand J Immunol. 2006 Feb;63(2):106-15. doi: 10.1111/j.1365-3083.2005.01715.x.
|
| 25 |
Ribavirin and alpha interferon enhance death receptor-mediated apoptosis and caspase activation in human hepatoma cells. Antimicrob Agents Chemother. 2003 Jun;47(6):1912-21. doi: 10.1128/AAC.47.6.1912-1921.2003.
|
| 26 |
Two distinct phases of virus-induced nuclear factor kappa B regulation enhance tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in virus-infected cells. J Biol Chem. 2003 May 16;278(20):18092-100. doi: 10.1074/jbc.M300265200. Epub 2003 Mar 13.
|
| 27 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 28 |
Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G910-22. doi: 10.1152/ajpgi.90672.2008. Epub 2009 Jan 22.
|
| 29 |
Characterization of ribavirin uptake systems in human hepatocytes. J Hepatol. 2010 Apr;52(4):486-92.
|
| 30 |
Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon--based regimens. Clin Pharmacol Ther. 2014 Feb;95(2):141-6. doi: 10.1038/clpt.2013.203. Epub 2013 Oct 4.
|
| 31 |
ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy--a genome-wide study of Japanese HCV virus patients. Gastroenterology. 2010 Oct;139(4):1190-7. doi: 10.1053/j.gastro.2010.06.071. Epub 2010 Jul 14.
|
| 32 |
A potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl-prelamin A in cells. J Biol Chem. 2008 Apr 11;283(15):9797-804. doi: 10.1074/jbc.M709629200. Epub 2008 Jan 28.
|
| 33 |
Company report (JMI Laboratories)
|
| 34 |
Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007 Nov;60(5):987-93.
|
| 35 |
Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br J Pharmacol. 2010 Jul;160(5):1224-33.
|
| 36 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 37 |
Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81.
|
| 38 |
Synthesis and antiviral activities of the major metabolites of the HIV protease inhibitor ABT-378 (Lopinavir). Bioorg Med Chem Lett. 2001 Jun 4;11(11):1351-3.
|
| 39 |
Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther. 2005 Feb;312(2):583-91.
|
| 40 |
Functional defect caused by the 4544G>A SNP in ABCC2: potential impact for drug cellular disposition. Pharmacogenet Genomics. 2011 Dec;21(12):884-93. doi: 10.1097/FPC.0b013e32834d672b.
|
| 41 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 42 |
Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12(4):489-500.
|
| 43 |
Heme oxygenase-1-derived bilirubin counteracts HIV protease inhibitor-mediated endothelial cell dysfunction. Free Radic Biol Med. 2016 May;94:218-29. doi: 10.1016/j.freeradbiomed.2016.03.003. Epub 2016 Mar 8.
|
| 44 |
Lopinavir inhibits meningioma cell proliferation by Akt independent mechanism. J Neurooncol. 2011 Feb;101(3):441-8. doi: 10.1007/s11060-010-0281-y. Epub 2010 Jul 2.
|
|
|
|
|
|
|