Details of the Drug
General Information of Drug (ID: DMEYLH9)
| Drug Name |
Ribavirin
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ribavirin; 36791-04-5; Tribavirin; Rebetol; Virazole; Ribavirine; Copegus; Vilona; Ribamide; Ribasphere; Ribamidil; Viramid; Ribamidyl; Ribavirinum; Ribavirina; Rebetron; Varazid; RTCA; Ribavirin Capsules; Ribavirinum [INN-Latin]; Ribavirine [INN-French]; Ribavirina [INN-Spanish]; ICN-1229; Rebretron; Virazid; Ribav; 1-beta-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide; DRG-0028; 1-beta-D-Ribofuranosyl-1H-1,2,4-triazole-3-carboxamide; UNII-49717AWG6K; Ribavirin (Copegus); Copegus; Cotronak; RBV; RTC; Ravanex; Ribacine; Ribovirin; Viramide; Virazide; R 9644; SCH 18908; C-Virin; Copegus (TN); Drug: Ribavirin; KS-1104; R-964; RG-964; Rebetol (TN); Ribasphere (TN); Ribavirin [USAN:INN]; Vilona (TN); Virazole (Ribavirin) Inhalation Solution; Virazole (TN); AA-504/07617051; Ro 20-9963/000; Ro-20-9963; Ribavirin (JAN/USP/INN); 1-beta-D-Ribofuranosyl-1,2,4-triazolo-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide; RBI034 (2-5A antisense compound) + Ribavirin
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Hepatitis B virusHepatitis C virus, RSV and other RNA/DNA viruses
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
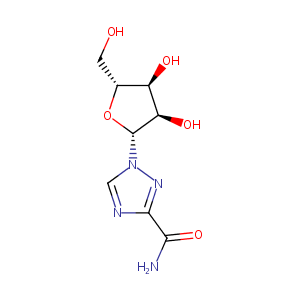 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 244.2 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.8 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ribavirin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Ribavirin FDA Label | ||||
| 3 | Proteomics of SARS-CoV-2-infected Host Cells Reveals Therapy Targets. Nature. 2020 May 14. doi: 10.1038/s41586-020-2332-7. | ||||
| 4 | SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016 Aug;14(8):523-34. | ||||
| 5 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 10 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 11 | Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006 Jan;57(1):8-13. | ||||
| 12 | Effects of dipyridamole coadministration on the pharmacokinetics of ribavirin in healthy volunteers. Drug Metab Pharmacokinet. 2013;28(5):406-10. | ||||
| 13 | Identification and functional analysis of variants in the human concentrative nucleoside transporter 2, hCNT2 (SLC28A2) in Chinese, Malays and Indians. Pharmacogenet Genomics. 2007 Sep;17(9):783-6. | ||||
| 14 | Kinetic study of anti-viral ribavirin uptake mediated by hCNT3 and hENT1 in Xenopus laevis oocytes. Biophys Chem. 2010 Mar;147(1-2):59-65. | ||||
| 15 | Transport of levovirin prodrugs in the human intestinal Caco-2 cell line. J Pharm Sci. 2006 Jun;95(6):1318-25. | ||||
| 16 | Functional analysis of purine nucleoside phosphorylase as a key enzyme in ribavirin metabolism. Drug Metab Pharmacokinet. 2014;29(2):211-4. | ||||
| 17 | Phosphorylation of ribavirin and viramidine by adenosine kinase and cytosolic 5'-nucleotidase II: Implications for ribavirin metabolism in erythrocytes. Antimicrob Agents Chemother. 2005 Jun;49(6):2164-71. | ||||
| 18 | Inhibition of cardiomyocyte differentiation of human induced pluripotent stem cells by Ribavirin: Implication for its cardiac developmental toxicity. Toxicology. 2020 Apr 15;435:152422. doi: 10.1016/j.tox.2020.152422. Epub 2020 Feb 26. | ||||
| 19 | Ribavirin and alpha interferon enhance death receptor-mediated apoptosis and caspase activation in human hepatoma cells. Antimicrob Agents Chemother. 2003 Jun;47(6):1912-21. doi: 10.1128/AAC.47.6.1912-1921.2003. | ||||
| 20 | Ribavirin inhibits colorectal cancer growth by downregulating PRMT5 expression and H3R8me2s and H4R3me2s accumulation. Toxicol Appl Pharmacol. 2021 Mar 15;415:115450. doi: 10.1016/j.taap.2021.115450. Epub 2021 Feb 9. | ||||
| 21 | Characterization of ribavirin uptake systems in human hepatocytes. J Hepatol. 2010 Apr;52(4):486-92. | ||||
| 22 | Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G910-22. doi: 10.1152/ajpgi.90672.2008. Epub 2009 Jan 22. | ||||
| 23 | Normal erythropoietin response in chronic hepatitis C patients with ribavirin-induced anaemia. Antivir Ther. 2003 Feb;8(1):57-63. | ||||
| 24 | Ribavirin inhibits the growth and ascites formation of hepatocellular carcinoma through downregulation of type I CARM1 and type II PRMT5. Toxicol Appl Pharmacol. 2022 Jan 15;435:115829. doi: 10.1016/j.taap.2021.115829. Epub 2021 Dec 14. | ||||
| 25 | Ribavirin augments doxorubicin's efficacy in human hepatocellular carcinoma through inhibiting doxorubicin-induced eIF4E activation. J Biochem Mol Toxicol. 2018 Jan;32(1). doi: 10.1002/jbt.22007. Epub 2017 Nov 7. | ||||
| 26 | Canadian Pharmacists Association. | ||||
