Details of the Drug Combinations
General Information of This Drug (ID: DMFKXDY)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ankebin; Antara; Controlip; Durafenat; Elasterate; Elasterin; FNF; Fenobeta; Fenobrate; Fenofanton; Fenofibrato; Fenofibratum; Fenogal; Fenoglide; Fenomax; Fenotard; Finofibrate; Fulcro; Lipanthyl; Lipantil; Liparison; Lipidex; Lipidil; Lipifen; Lipirex; Lipoclar; Lipofen; Lipofene; Liposit; Lipsin; Lofibra; Luxacor; Nolipax; Pharmavit; Phenofibrate; Procetofen; Procetofene; Proctofene; Protolipan; Secalip; Sedufen; Supralip; Tricor; Triglide; AbZ Brand of Procetofen; Abbott Brand of Procetofen; Aliud Brand of Procetofen; Antara Micronized Procetofen; Anto Brand of Procetofen; Apo Feno Micro; Apo Fenofibrate; Apotex Brand of Procetofen; Azupharma Brand of Procetofen; Betapharm Brand of Procetofen; Bouchara Brand of Procetofen; Ct Arzneimittel Brand of Procetofen; Fenofibrat AL; Fenofibrat AZU; Fenofibrat AbZ; Fenofibrat FPh; Fenofibrat Heumann; Fenofibrat Hexal; Fenofibrat Stada; Fenofibrat ratiopharm; Fenofibrat von ct; Fenofibrate Debat; Fenofibrate MSD; Fournier Brand of Procetofen; GNR Pharma Brand of Procetofen; Gate Brand of Procetofen; Gen Fenofibrate; Genpharm Brand of Procetofen; Heumann Brand of Procetofen; Hexal Brand of Procetofen; Knoll Brand of Procetofen; Lichtenstein Brand of Procetofen; Lipidil Micro; Lipidil Supra; Lipidil Ter; MTW Brand of Procetofen; MTW Fenofibrat; Merck dura Brand of Procetofen; Novartis Brand of Procetofen; Novo Fenofibrate; Novopharm Brand of Procetofen; Nu Fenofibrate; Nu Pharm Brand of Procetofen; PMS Fenofibrate Micro;Pharmascience Brand of Procetofen; Procetofen Reliant Brand; Q Pharm Brand of Procetofen; Ratiopharm Brand of Procetofen; Reliant Brand of Procetofen; Schering Plough Brand of Procetofen; Stadapharm Brand of Procetofen; United Drug Brand of Procetofen; F 6020; LF 178; LF178; AZU, Fenofibrat; Antara (TN); Antara (micronized); Apo-Fenofibrate; CIP-Fenofibrate; Ct-Arzneimittel Brand of Procetofen; Debat, Fenofibrate; FENOFIBRATE (MICRONIZED); Fenofibrat-ratiopharm; Fenofibrate IDD-P; Fenofibrate [INN:BAN]; Fenofibrato [INN-Spanish]; Fenofibratum [INN-Latin]; Fenogal (TN); GNR-Pharma Brand of Procetofen; GRS-027; Gen-Fenofibrate; Heumann, Fenofibrat; Hexal, Fenofibrat; LCP-Feno; LCP-FenoChol; LF-178; Lipanthyl (TN); Lipantil (TN); Lipidil (TN); Lipidil-Ter; Lipofen (TN); Lofibra (TN); MTW-Fenofibrat; Micronized Procetofen, Antara; Novo-Fenofibrate; Nu-Fenofibrate; Nu-Pharm Brand of Procetofen; PMS-Fenofibrate Micro; Procetofen, Antara Micronized; Q-Pharm Brand of Procetofen; Schering-Plough Brand of Procetofen; Stada, Fenofibrat; TRICOR (MICRONIZED); Tricor (TN); Triglide (TN); Trilipix (TN); Apo-Feno-Micro; Fenocor-67 (TN); Fenofibrate (JAN/INN); Isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate; Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionate; Isopropyl 2-(p-(p-chlorobenzoyl)phenoxy)-2-methylpropionate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antilipemic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
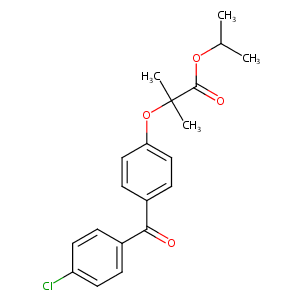 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
9 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
