Details of the Drug
General Information of Drug (ID: DMFKXDY)
| Drug Name |
Fenofibrate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ankebin; Antara; Controlip; Durafenat; Elasterate; Elasterin; FNF; Fenobeta; Fenobrate; Fenofanton; Fenofibrato; Fenofibratum; Fenogal; Fenoglide; Fenomax; Fenotard; Finofibrate; Fulcro; Lipanthyl; Lipantil; Liparison; Lipidex; Lipidil; Lipifen; Lipirex; Lipoclar; Lipofen; Lipofene; Liposit; Lipsin; Lofibra; Luxacor; Nolipax; Pharmavit; Phenofibrate; Procetofen; Procetofene; Proctofene; Protolipan; Secalip; Sedufen; Supralip; Tricor; Triglide; AbZ Brand of Procetofen; Abbott Brand of Procetofen; Aliud Brand of Procetofen; Antara Micronized Procetofen; Anto Brand of Procetofen; Apo Feno Micro; Apo Fenofibrate; Apotex Brand of Procetofen; Azupharma Brand of Procetofen; Betapharm Brand of Procetofen; Bouchara Brand of Procetofen; Ct Arzneimittel Brand of Procetofen; Fenofibrat AL; Fenofibrat AZU; Fenofibrat AbZ; Fenofibrat FPh; Fenofibrat Heumann; Fenofibrat Hexal; Fenofibrat Stada; Fenofibrat ratiopharm; Fenofibrat von ct; Fenofibrate Debat; Fenofibrate MSD; Fournier Brand of Procetofen; GNR Pharma Brand of Procetofen; Gate Brand of Procetofen; Gen Fenofibrate; Genpharm Brand of Procetofen; Heumann Brand of Procetofen; Hexal Brand of Procetofen; Knoll Brand of Procetofen; Lichtenstein Brand of Procetofen; Lipidil Micro; Lipidil Supra; Lipidil Ter; MTW Brand of Procetofen; MTW Fenofibrat; Merck dura Brand of Procetofen; Novartis Brand of Procetofen; Novo Fenofibrate; Novopharm Brand of Procetofen; Nu Fenofibrate; Nu Pharm Brand of Procetofen; PMS Fenofibrate Micro;Pharmascience Brand of Procetofen; Procetofen Reliant Brand; Q Pharm Brand of Procetofen; Ratiopharm Brand of Procetofen; Reliant Brand of Procetofen; Schering Plough Brand of Procetofen; Stadapharm Brand of Procetofen; United Drug Brand of Procetofen; F 6020; LF 178; LF178; AZU, Fenofibrat; Antara (TN); Antara (micronized); Apo-Fenofibrate; CIP-Fenofibrate; Ct-Arzneimittel Brand of Procetofen; Debat, Fenofibrate; FENOFIBRATE (MICRONIZED); Fenofibrat-ratiopharm; Fenofibrate IDD-P; Fenofibrate [INN:BAN]; Fenofibrato [INN-Spanish]; Fenofibratum [INN-Latin]; Fenogal (TN); GNR-Pharma Brand of Procetofen; GRS-027; Gen-Fenofibrate; Heumann, Fenofibrat; Hexal, Fenofibrat; LCP-Feno; LCP-FenoChol; LF-178; Lipanthyl (TN); Lipantil (TN); Lipidil (TN); Lipidil-Ter; Lipofen (TN); Lofibra (TN); MTW-Fenofibrat; Micronized Procetofen, Antara; Novo-Fenofibrate; Nu-Fenofibrate; Nu-Pharm Brand of Procetofen; PMS-Fenofibrate Micro; Procetofen, Antara Micronized; Q-Pharm Brand of Procetofen; Schering-Plough Brand of Procetofen; Stada, Fenofibrat; TRICOR (MICRONIZED); Tricor (TN); Triglide (TN); Trilipix (TN); Apo-Feno-Micro; Fenocor-67 (TN); Fenofibrate (JAN/INN); Isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate; Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionate; Isopropyl 2-(p-(p-chlorobenzoyl)phenoxy)-2-methylpropionate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antilipemic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
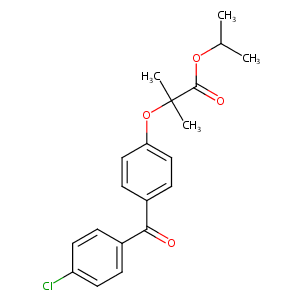 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 360.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Coronary atherosclerosis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Fenofibrate
Coadministration of a Drug Treating the Disease Different from Fenofibrate (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Fenofibrate FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7186). | ||||
| 3 | Balfour JA, McTavish D, Heel RC: Fenofibrate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in dyslipidaemia. Drugs. 1990 Aug;40(2):260-90. doi: 10.2165/00003495-199040020-00007. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | FDA Approved Drug Products: Fenofibrate Oral Tablets | ||||
| 6 | Chapman MJ: Pharmacology of fenofibrate. Am J Med. 1987 Nov 27;83(5B):21-5. doi: 10.1016/0002-9343(87)90867-9. | ||||
| 7 | Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet. 1998 Feb;34(2):155-62. doi: 10.2165/00003088-199834020-00003. | ||||
| 8 | Simmons RE, Hjelle JT, Mahoney C, Deftos LJ, Lisker W, Kato P, Rabkin R: Renal metabolism of calcitonin. Am J Physiol. 1988 Apr;254(4 Pt 2):F593-600. doi: 10.1152/ajprenal.1988.254.4.F593. | ||||
| 9 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 10 | Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000 Jun 2;275(22):16638-42. | ||||
| 11 | Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet. 1998 Feb;34(2):155-62. | ||||
| 12 | Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004 Nov;32(11):1201-8. | ||||
| 13 | Transcriptomics hit the target: monitoring of ligand-activated and stress response pathways for chemical testing. Toxicol In Vitro. 2015 Dec 25;30(1 Pt A):7-18. | ||||
| 14 | Transcriptomic analysis of untreated and drug-treated differentiated HepaRG cells over a 2-week period. Toxicol In Vitro. 2015 Dec 25;30(1 Pt A):27-35. | ||||
| 15 | Linalool is a PPARalpha ligand that reduces plasma TG levels and rewires the hepatic transcriptome and plasma metabolome. J Lipid Res. 2014 Jun;55(6):1098-110. | ||||
| 16 | AMPK-dependent signaling modulates the suppression of invasion and migration by fenofibrate in CAL 27 oral cancer cells through NF-B pathway. Environ Toxicol. 2016 Jul;31(7):866-76. doi: 10.1002/tox.22097. Epub 2014 Dec 24. | ||||
| 17 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 18 | Canadian Pharmacists Association. | ||||
| 19 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 20 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 21 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 22 | Asplund K, Wiholm BE, Lithner F "Glibenclamide-associated hypoglycaemia: a report on 57 cases." Diabetologia 24 (1983): 412-7. [PMID: 6411511] | ||||
| 23 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 24 | Hedaya MA, El-Afify DR, El-Maghraby GM "The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers." Biopharm Drug Dispos 27 (2006): 103-10. [PMID: 16372380] | ||||
| 25 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 26 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 27 | Adkins JC, Faulds D "Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia." Drugs 54 (1997): 615-33. [PMID: 9339964] | ||||
| 28 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 29 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 30 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 31 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 32 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 33 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 34 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
