Details of the Drug Combinations
General Information of This Drug (ID: DMG592Q)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Vesicare; Vesikur; Solifenacin succinate; Solifenacin succinate [USAN]; YM 905; Solifenacin (INN); Solifenacin [INN:BAN]; Vesicare (TN); YM-53705; YM-67905; YM-905; Quinuclidin-3'-yl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylatemonosuccinate; [(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate; Butanedioic acid, compd with (1S)-(3R)-1-azabicyclo(2.2.2)oct-3-yl 3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate (1:1); Butanedioic acid, cmpd. with (1S)-(3R)-1-azabicyclo(2.2.2)oct-3-yl 3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate (1:1); 1-azabicyclo[2.2.2]oct-8-yl (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate; 1-azabicyclo[2.2.2]octan-3-yl (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate; 2(1H)-Isoquinolinecarboxylic acid, 3,4-dihydro-1-phenyl-, (3R)-1-azabicyclo(2.2.2)oct-3-yl ester, (1S)-, butanedioate (1:1)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antispasmodics
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
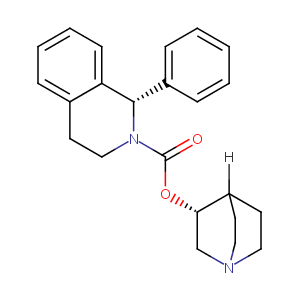 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
