| 1 |
ClinicalTrials.gov (NCT02294396) Postmarketing Study to Evaluate add-on Therapy With Anticholinergics in Patients With Overactive Bladder (OAB) on Mirabegron.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7483).
|
| 3 |
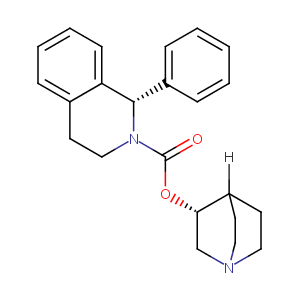
Solifenacin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7445).
|
| 5 |
Comparison of muscarinic receptor selectivity of solifenacin and oxybutynin in the bladder and submandibular gland of muscarinic receptor knockout ... Eur J Pharmacol. 2009 Aug 1;615(1-3):201-6.
|
| 6 |
Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009;48(5):281-302.
|
| 7 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 8 |
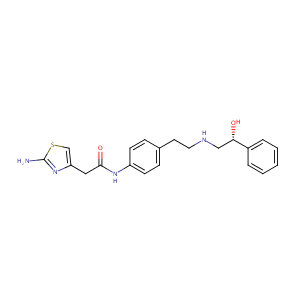
Role of cytochrome p450 isoenzymes 3A and 2D6 in the in vivo metabolism of mirabegron, a beta-3-adrenoceptor agonist. Clin Drug Investig. 2013 Jun;33(6):429-40.
|
| 9 |
ClinicalTrials.gov (NCT01745094) A Study to Evaluate the Effect of Mirabegron + Solifenacin in Overactive Bladder Patients
|
|
|
|
|
|
|