Details of the Drug Combinations
General Information of This Drug (ID: DMIZKOP)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
thiotepa; THIO-TEPA; 52-24-4; Triethylenethiophosphoramide; Thiophosphamide; Tiofosyl; Tiofosfamid; Thiofozil; Tiofozil; Thioplex; Tespamin; Oncotepa; Tespamine; Girostan; Thiotef; Stepa; Tio-tef; Thio-Tep; Oncotiotepa; Tifosyl; Oncothio-tepa; TESPA; TSPA; Thio-tepa S; Ledertepa; Thiotriethylenephosphoramide; TIO TEF; N,N',N''-Triethylenethiophosphoramide; Tris(1-aziridinyl)phosphine sulfide; Thiophosphamidum; 1,1',1''-phosphorothioyltriaziridine; Triethylene thiophosphoramide; Triaziridinylphosphine sulfide
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
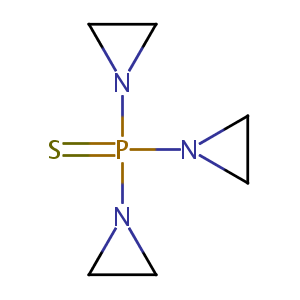 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
10 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
