| 1 |
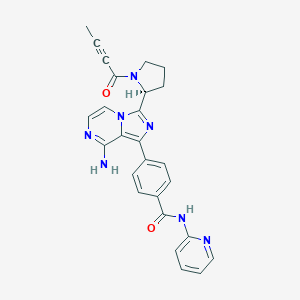
ClinicalTrials.gov (NCT05021770) Orelabrutinib in Combination With Thiotepa in Refractory and Relapsed Primary CNS Lymphoma
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 210259
|
| 3 |
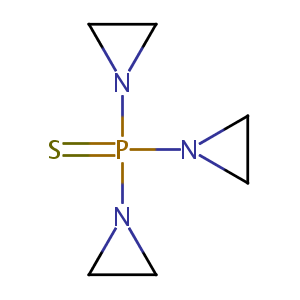
2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
ClinicalTrials.gov (NCT04346199) Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19.. U.S. National Institutes of Health.
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7622).
|
| 7 |
Thiotepa FDA Label
|
| 8 |
AstraZeneca initiates CALAVI clinical trial with Calquence against COVID-19
|
| 9 |
FDA label of Acalabrutinib. The 2020 official website of the U.S. Food and Drug Administration.
|
| 10 |
Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020 Jun 5;5(48):eabd0110. doi: 10.1126/sciimmunol.abd0110. Epub 2020 Jun 5.
|
| 11 |
Functional assessment of the effects of CYP3A4 variants on acalabrutinib metabolism in vitro. Chem Biol Interact. 2021 Aug 25;345:109559. doi: 10.1016/j.cbi.2021.109559. Epub 2021 Jun 18.
|
| 12 |
Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000 Jun;7(6):574-86. doi: 10.1038/sj.cdd.4400688.
|
| 13 |
Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thiotepa to TEPA. Cancer Chemother Pharmacol. 2002 Jun;49(6):461-7.
|
| 14 |
Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys. 1999 Jun 1;366(1):89-94.
|
| 15 |
Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa. Br J Clin Pharmacol. 2009 Jan;67(1):50-60. doi: 10.1111/j.1365-2125.2008.03321.x. Epub 2008 Nov 17.
|
| 16 |
The naturally occurring cytochrome P450 (P450) 2B6 K262R mutant of P450 2B6 exhibits alterations in substrate metabolism and inactivation. Drug Metab Dispos. 2005 Jun;33(6):795-802.
|
| 17 |
Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 2009 Jun;7(6):897-906. doi: 10.1158/1541-7786.MCR-08-0519. Epub 2009 May 26.
|
| 18 |
Relations between polymorphisms in drug-metabolising enzymes and toxicity of chemotherapy with cyclophosphamide, thiotepa and carboplatin. Pharmacogenet Genomics. 2008 Nov;18(11):1009-15. doi: 10.1097/FPC.0b013e328313aaa4.
|
| 19 |
[Establishment of MRP-overexpression subline of bladder carcinoma and its MDR phenotype]. Zhonghua Zhong Liu Za Zhi. 2000 Jul;22(4):273-5.
|
| 20 |
Protection of mammalian cells against chemotherapeutic agents thiotepa, 1,3-N,N'-bis(2-chloroethyl)-N-nitrosourea, and mafosfamide using the DNA base excision repair genes Fpg and alpha-hOgg1: implications for protective gene therapy applications. J Pharmacol Exp Ther. 2001 Mar;296(3):825-31.
|
| 21 |
Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999 Apr 1;18(13):2241-51. doi: 10.1038/sj.onc.1202526.
|
|
|
|
|
|
|