Details of the Drug Combinations
General Information of This Drug (ID: DMMIQ7G)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Astende; Cirantan; Cresadex; Creston; Crestor; Provisacor; Razel; Rosedex; Rosimol; Rosumed; Rosustatin; Rosuvas; Rosuvast; Rosvel; Rovartal; Simestat; Sinlip; Vivacor; Rosuvastatin [INN]; Rosuvastatin calcium; Rosuvastatin calcium [USAN]; Rosuvastatin hemicalcium; S 4522; ZD 4522; ZD4522; AZD-4522; Creston (TN);Crestor (TN); Pyrimidine Compound, 26; Rosuvastatin (INN); S-4522; ZD 4522, calcium salt; ZD-4522; Rosuvastatin calcium (JAN/USAN); Bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhelpt-6-enoic acid] calcium salt; Calcium (E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate; (3R,5S,6E)-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid; (3R,5S,6E)-7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic acid; (E,3R,5R)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid; (E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid; (S-((R*,S*-(E)))-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl) amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid, calcium salt (2:1); (S-(R*,S*-(E)))-7-(4-(4-Fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid, calcium salt (2:1); 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, (3R,5S,6E); 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, calcium salt (2:1), (3R,5S,6E)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
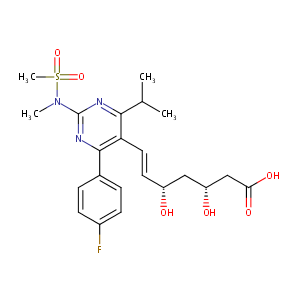 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
31 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
