Details of the Drug Combinations
General Information of This Drug (ID: DMMQ8ZG)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aisemide; Aldalix; Aldic; Aluzine; Anfuramaide; Aquarid; Aquasin; Arasemide; Beronald; Bioretic; Cetasix; Depix; Desal; Desdemin; Dirine; Disal; Discoid; Disemide; Diural; Diurapid; Diurin; Diurolasa; Diusemide; Diusil; Diuzol; Dranex; Dryptal; Durafurid; Edemid; Edenol; Eliur; Endural; Errolon; Eutensin; FUN; Farsix; Fluidrol; Fluss; Franyl; Frumex; Frumide; Frusedan; Frusema; Frusemid; Frusemide; Frusemin; Frusenex; Frusetic; Frusid; Fulsix; Fuluvamide; Fuluvamine; Fulvamide; Furanthril; Furanthryl; Furantral; Furantril; Furanturil; Furesis; Furetic; Furex; Furfan; Furix; Furmid; Furobeta; Furocot; Furodiurol; Furodrix; Furomen; Furomex; Furorese; Furosan; Furose; Furosedon; Furosemid; Furosemida; Furosemidu; Furosemidum; Furosemix; Furoside; Furosifar; Furosix; Furoter; Furovite; Fursemid; Fursemida; Fursemide; Fursol; Fusid; Golan; Hissuflux; Hydrex; Hydro; Hydroled; Impugan; Jenafusid; Katlex; Kofuzon; Kolkin; Kutrix; Lasemid; Lasex; Lasiletten; Lasilix; Lasix; Laxur; Lazix; Liside; Logirene; Lowpston; Lowpstron; Luscek; Macasirool; Marsemide; Mirfat; Mita; Moilarorin; Myrosemide; Nadis; Nelsix; Novosemide; Odemase; Odemex; Oedemex; Prefemin; Profemin; Promedes; Promide; Protargen; Puresis; Radisemide; Radonna; Radouna; Retep; Rosemide; Rosis; Rusyde; Salinex; Salix; Salurex; Salurid; Seguril; Selectofur; Sigasalur; Spirofur; Synephron; Transit; Trofurit; Uremide; Uresix; Urian; Uridon; Uritol; Urosemide; Vesix; Yidoli; Zafimida; FUROSEMIDE USP; Fu sid; Furosemide Monohydrochloride; Furosemide Monosodium Salt; Furosemidu [Polish]; Lasix Retard; Lasix Special; Less Diur; Polysquall A; Sal diureticum; F0182; F4381_SIGMA; LB 502; Aisemide (TN); Apo-Frusemide; Apo-Furosemide; Beronald (TN); Desdemin (TN); Discoid (TN); Diumide-K; Diural (TN); Diurapid (TN); Dryptal (TN); Durafurid (TN); Errolon (TN); Eutensin (TN); Frudix (TN); Frusetic (TN); Frusid (TN); Fulsix (TN); Fuluvamide (TN); Furesis (TN); Furix (TN); Furo-Basan; Furo-puren; Furomide M.D; Furosedon (TN); Furosemida [INN-Spanish]; Furosemide (mita); Furosemidum [INN-Latin]; Hoe-058A; Hydro-rapid; Impugan (TN); Katlex (TN); LB-502; Lasilix (TN); Lasix (TN); Lodix (TN); Lowpston (TN); Macasirool (TN); Mirfat (TN); Neo-renal; Nicorol (TN); Odemase (TN); Oedemex (TN); Profemin (TN); Rosemide (TN); Rusyde (TN); Salix (TN); Salix (brand of furosemide); Trofurit (TN); Urex (TN); Urex-M; Apo-Furosemide (TN); Furo-Puren (TN); Furomide M.D.; Furosemide [USAN:INN:JAN]; Hydro-rapid(TN); Lasix, Frusemide, Furosemide; Furosemide (JP15/USP/INN); Chlor-N-(2-furylmethyl)-5-sulfamylanthranilsaeure; Chlor-N-(2-furylmethyl)-5-sulfamylanthranilsaeure [German]; 2-Furfurylamino-4-chloro-5-sulfamoylbenzoic acid; 4-Chloro-5-sulfamoyl-N-furfuryl-anthranilic acid; 4-Chloro-N-(2-furylmethyl)-5-sulfamoylanthranilic acid; 4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid; 4-chloro-2-(furan-2-ylmethylamino)-5-sulfamoylbenzoic acid; 4-chloro-2-[(furan-2-ylmethyl)amino]-5-sulfamoylbenzoic acid
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Diuretics
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
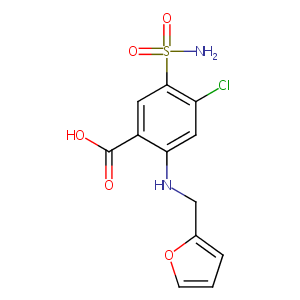 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
16 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
