Details of the Drug Combinations
General Information of This Drug (ID: DMOLNHF)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alkeran; Levofalan; Levofolan; Levopholan; Melfalan; Melfalano; Melphalanum; Alanine Nitrogen Mustard; Phenylalanine mustard; Phenylalanine nitrogen mu stard; Phenylalanine nitrogen mustard; AY3360000; CB 3025; ALKERAN (TN); Alkeran (TN); At-290; CB-3025; L-PAM; L-Phenylalanine mustard; L-Sarcolysin; L-Sarcolysine; L-Sarkolysin; Melfalano [INN-Spanish]; Melphalanum [INN-Latin]; SK-15673; TRANSGENIC MODEL EVALUATION (MELPHALAN); MELPHALAN (SEE ALSO TRANSGENIC MODEL EVALUATION (MELPHALAN)); P-L-Sarcolysin; P-L-sarcolysine; TRANSGENIC LEP (MELPHALAN) (SEE ALSO MELPHALAN); Melphalan (JP15/USP/INN); Melphalan [USAN:INN:BAN:JAN]; P-Bis(beta-chloroethyl)aminophenylalanine; P-N-Di(chloroethyl)aminophenylalanine; P-N-di(chloroethyl)aminophenylala nine; P-Di-(2-chloroethyl)amino-L-phenylalanine; P-N-Bis(2-chloroethyl)amino-L-phenylalanine; L-3-(p-(Bis(2-chloroethyl)amino)phenyl)alanine; L-3-(para-(Bis(2-chloroethyl)amino)phenyl)alanine; P-N,N-bis(2-chloroethyl)amino-L-phenylalanine; (2S)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoic acid; (2s)-2-amino-3-(4-[bis(2-chloroethyl)amino]phenyl)propanoic acid; 3-(p-(Bis(2-chloroethyl)amino)phenyl)-L-alanine; 3-(p-(Bis(2-chloroethyl)amino)phenyl)alanine; 3-p-(Di(2-chloroethyl)amino)-phenyl-L-alanine; 4-(Bis(2-chloroethyl)amino)-L-phenylalanine; 4-[Bis(2-chloroethyl)amino]-L-phenylalanine; 4-[Bis-(2-chloroethyl)amino]-L-phenylalanine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
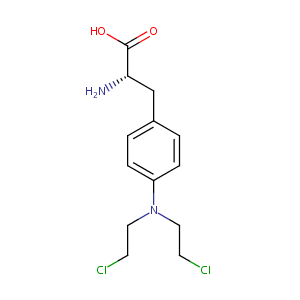 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
