Details of the Drug Combinations
General Information of This Drug (ID: DMRF9YK)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
864070-44-0; JARDIANCE; BI 10773; UNII-HDC1R2M35U; Empagliflozin (BI 10773); BI-10773; BI10773; HDC1R2M35U; CHEBI:82720; 1-chloro-4-(glucopyranos-1-yl)-2-(4-(tetrahydrofuran-3-yloxy)benzyl)benzene; AK160980; (1S)-1,5-anhydro-1-(4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl)-D-glucitol; (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
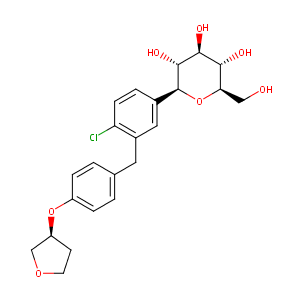 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
13 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2 Approved Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
