Details of the Drug Combinations
General Information of This Drug (ID: DMRL3AB)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pepcid; famotidine; 76824-35-6; Pepcid AC; Pepcidine; Quamatel; Gastridin; Famodil; Dispromil; Pepdine; Digervin; Gaster; Fluxid; Pepdul; Pepcid RPD; Famulcer; Supertidine; Pepcidina; Fagastine; Whitidin; Farmotex; Peptifam; Ferotine; Dispronil; Tairal; Sigafam; Famtac; Durater; Yamarin; Pepzan; Famoxal; Evatin; Weimok; Pepdif; Fudone; Fanosin; Fanobel; Duovel; Fibonel; Fadine; Dipsin; Ganor; Fadin; Dinul; Fanox; Fadyn; Famox; Famo; Nu-Famotidine; Pepcidin Rapitab; Sedanium-R; Dibrit 40; Famotidinum [Latin]; PEPCID; Famotidina [Spanish]; Apogastine; Antodine; Bestidine; Amfamox; Blocacid; Brolin; Cepal; Confobos; Cronol; Cuantin; Famocid; Famodar; Famodin; Famodine; Famogard; Famonit; Famopsin; Famos; Famosan; Famotal; Famotep; Famotin; Famovane; Famowal; Gastridan; Gastrion; Gastro; Gastrodomina; Gastrofam; Gastropen; Gastrosidin; Hacip; Huberdina; Ingastri; Invigan; Lecedil; Logos; Mensoma; Midefam; Mosul; Motiax; Muclox; Neocidine; Nevofam; Notidin; Nulceran; Nulcerin; Panalba; Pepcidac; Pepcidin; Pepfamin; Peptan; Peptidin; Purifam; Quamtel; Renapepsa; Restadin; Rogasti; Rubacina; Tamin; Tipodex; Topcid; Ulcatif; Ulceprax; Ulcofam; Ulfagel; Ulfam; Ulfamid; Ulfinol; Ulgarine; Vagostal; FAMOTIDINE PRESERVATIVE FREE; FAMOTIDINE PRESERVATIVE FREE IN PLASTIC CONTAINER; Mylanta AR; PEPCID COMPLETE; PEPCID PRESERVATIVE FREE; PEPCID PRESERVATIVE FREE IN PLASTIC CONTAINER; Pepcid AC Gelcaps; PepcidRPD; F 6889; F0530; L 643341; MK 208; YM 11170; Apo-Famotidine; HS-0054; MK-208; Novo-Famotidine; Pepcid (TN); Pepcidine (TN); YM-11170; YM-1170; Famotidine [USAN:BAN:INN:JAN]; Propanimidamide, 3-[[[2-[aminoiminomethyl)amino]-4-thiazoyl]methyl]thio]-N-(aminosulfonyl); N'-(Aminosulfonyl)-3-([2-(diaminomethyleneamino)-4-thiazolyl]methylthio)propanamidine; (1-Amino-3-(((2-((diaminomethylene)amino)-4-thiazolyl)methyl)thio)propylidene)sulfamide; (1Z)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-N'-sulfamoylpropanimidamide; (1Z)-N'-(aminosulfonyl)-3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)thio]propanimidamide; 3-(2-Guanidinothiazol-4-ylmethylthio)-N1-sulfamoylpropionamide; 3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-N-sulfamoylpropanimidamide; 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-N'-sulfamoylpro; 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-N'-sulfamoylpropanimidamide
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiulcer Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
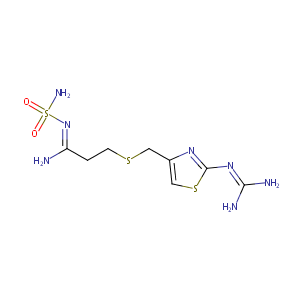 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
