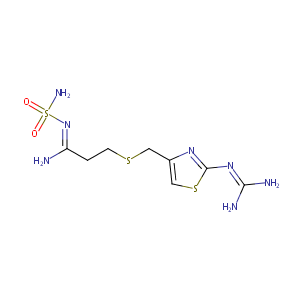
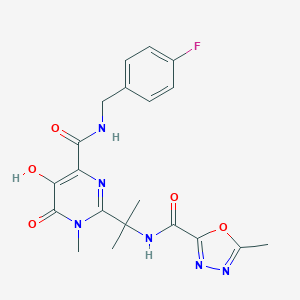
| 1 |
ClinicalTrials.gov (NCT01000818) A Study to Evaluate the Effect of Famotidine and Omeprazole on MK0518 (Raltegravir) Pharmacokinetics in Human Immunodeficiency Virus (HIV)-Infected Patients (0518-054)
|
| 2 |
Famotidine FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7074).
|
| 4 |
ClinicalTrials.gov (NCT04370262) Multi-site Adaptive Trials Using Hydroxycholoroquine for COVID-19. U.S. National Institutes of Health.
|
| 5 |
2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9.
|
| 6 |
Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J Invest Dermatol. 2001 Feb;116(2):261-5.
|
| 7 |
A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005 Oct;315(1):337-45.
|
| 8 |
Different transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A). Eur J Pharmacol. 2004 Oct 25;503(1-3):25-30.
|
| 9 |
Initial 48-hour acid inhibition by intravenous infusion of omeprazole, famotidine, or both in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2006 Nov;80(5):539-48.
|
| 10 |
Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J Pharmacol Exp Ther. 2005 Dec;315(3):1288-97.
|
| 11 |
Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17.
|
| 12 |
Hyperprolactinaemia during famotidine therapy. Lancet. 1993 Oct 2;342(8875):868. doi: 10.1016/0140-6736(93)92729-d.
|
| 13 |
Effects of three H2-receptor antagonists (cimetidine, famotidine, ranitidine) on serum gastrin level. Int J Clin Pharmacol Res. 2002;22(2):29-35.
|
| 14 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 15 |
Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010 Sep 30;467(7315):596-9. doi: 10.1038/nature09454.
|
| 16 |
Famotidine has a neuroprotective effect on MK-801 induced toxicity via the Akt/GSK-3/-catenin signaling pathway in the SH-SY5Y cell line. Chem Biol Interact. 2019 Dec 1;314:108823. doi: 10.1016/j.cbi.2019.108823. Epub 2019 Sep 26.
|
| 17 |
Increased immunoreactivity and concentration of basic fibroblast growth factor in lansoprazole-treated gastric mucosa. J Clin Gastroenterol. 1995;21 Suppl 1:S24-9.
|
| 18 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 19 |
Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3). Mol Pharm. 2015 Dec 7;12(12):4301-10.
|
| 20 |
Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrob Agents Chemother. 2012 Jun;56(6):2959-66. doi: 10.1128/AAC.05424-11. Epub 2012 Feb 27.
|
| 21 |
Successful tacrolimus treatment following renal transplant in a HIV-infected patient with raltegravir previously treated with a protease inhibitor based regimen. Drug Metabol Drug Interact. 2011;26(3):139-41.
|
| 22 |
Exposure-related effects of atazanavir on the pharmacokinetics of raltegravir in HIV-1-infected patients. Ther Drug Monit. 2010 Dec;32(6):782-6.
|
|
|
|
|
|
|