| 1 |
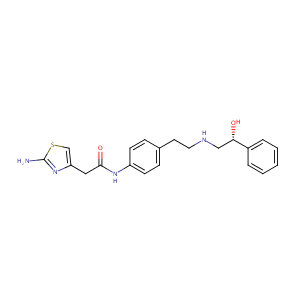
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7445).
|
| 2 |
ClinicalTrials.gov (NCT01478568) To Evaluate the Effect of Mirabegron (YM178) on Blood Levels of Desipramine When They Are Taken Together
|
| 3 |
ClinicalTrials.gov (NCT00776516) Pharmacokinetic Interaction Study to Assess the Effect of Repeat Doses of Rifampin on Mirabegron (YM178) in Healthy Volunteers
|
| 4 |
ClinicalTrials.gov (NCT01745094) A Study to Evaluate the Effect of Mirabegron + Solifenacin in Overactive Bladder Patients
|
| 5 |
ClinicalTrials.gov (NCT01489696) A Study to Evaluate Cardiovascular Interactions Between Mirabegron and Tamsulosin
|
| 6 |
ClinicalTrials.gov (NCT02095665) Ureteral Stent-related Pain and Mirabegron (SPAM) Trial
|
| 7 |
ClinicalTrials.gov (NCT02294396) Postmarketing Study to Evaluate add-on Therapy With Anticholinergics in Patients With Overactive Bladder (OAB) on Mirabegron.
|
| 8 |
ClinicalTrials.gov (NCT05051436) The Effects of Mirabegron and Tadalafil on Glucose Tolerance in Prediabetics
|
| 9 |
ClinicalTrials.gov (NCT02656173) A Phase 4 Study to Evaluate the Efficacy, Safety, and Tolerability of Mirabegron in Male Subjects With Overactive Bladder (OAB) Symptoms, While Taking the Alpha Blocker for Benign Prostatic Hypertrophy (BPH)
|
|
|
|
|
|
|