Details of the Drug Combinations
General Information of This Drug (ID: DMS3GX2)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Kinzal; Kinzalmono; Micardis; Pritor; Abbott brand of telmisartan; Boehringer Ingelheim brand of telmisartan; Glaxo Wellcome brand of telmisartan; GlaxoSmithKline brand of telmisartan; BIBR 277; BIBR 277SE; BIBR-277; BIBR-277SE; Bay 68-9291; Micardis (TN); Telmisartan [USAN:INN]; YM-086; BIBR-277-SE; Telmisartan (JAN/USAN/INN); Micardis, Targit, Temax, BIBR277, Telmisartan; 2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propylbenzimidazol-1-yl]methyl]phenyl]benzoic acid; 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidazol)-1'-yl)methyl)-(1,1'-biphenyl)-2-carboxylic acid; 4'-((4-Methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl)methyl)-2-biphenylcarboxylic acid; 4'-((4-mehtyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimmidazolyl)methyl)-2-biphenylcarboxylic acid; 4'-[(1,4'-dimethyl-2'propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid; 4'-[(1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-bibenzimidazol-3'-yl)methyl][1,1'-biphenyl]-2-carboxylic acid; 4'-[(1,7'-dimethyl-2'-propyl-1H,3'H-2,5'-bibenzimidazol-3'-yl)methyl]biphenyl-2-carboxylic acid
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
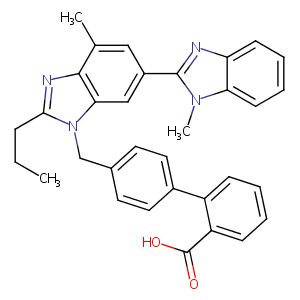 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
12 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
