| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
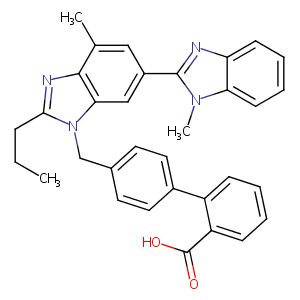
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 592).
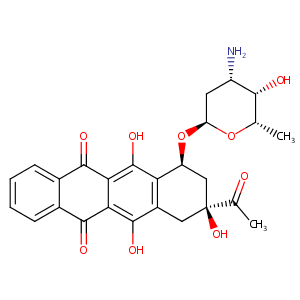
|
| 3 |
Telmisartan FDA Label
|
| 4 |
ClinicalTrials.gov (NCT04359953) Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on the Survival of Hospitalized Elderly Patients With COVID-19. U.S. National Institutes of Health.
|
| 5 |
Idarubicin FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7083).
|
| 7 |
Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009 Jun;50(6):1226-35.
|
| 8 |
Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020 Apr 3.
|
| 9 |
Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006 Jul;34(7):1109-15.
|
| 10 |
Establishment of a set of double transfectants coexpressing organic anion transporting polypeptide 1B3 and hepatic efflux transporters for the characterization of the hepatobiliary transport of telmisartan acylglucuronide. Drug Metab Dispos. 2008 Apr;36(4):796-805.
|
| 11 |
The impact of pharmacogenetics of metabolic enzymes and transporters on the pharmacokinetics of telmisartan in healthy volunteers. Pharmacogenet Genomics. 2011 Sep;21(9):523-30.
|
| 12 |
Characterization of in vitro glucuronidation clearance of a range of drugs in human kidney microsomes: comparison with liver and intestinal glucuronidation and impact of albumin. Drug Metab Dispos. 2012 Apr;40(4):825-35.
|
| 13 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 14 |
Toxicological evaluation of acyl glucuronides utilizing half-lives, peptide adducts, and immunostimulation assays. Toxicol In Vitro. 2015 Dec 25;30(1 Pt B):241-9.
|
| 15 |
Inhibitory effects of antihypertensive drugs on human cytochrome P450 2J2 activity: Potent inhibition by azelnidipine and manidipine. Chem Biol Interact. 2019 Jun 1;306:1-9.
|
| 16 |
Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005 May 15;4:6. doi: 10.1186/1475-2840-4-6.
|
| 17 |
Improvement of endothelial function in patients with hypertension and type 2 diabetes after treatment with telmisartan. Hypertens Res. 2010 Aug;33(8):796-801. doi: 10.1038/hr.2010.107. Epub 2010 Jun 17.
|
| 18 |
Effect of telmisartan on ambulatory blood pressure monitoring, plasma brain natriuretic peptide, and oxidative status of serum albumin in hemodialysis patients. Hypertens Res. 2005 Dec;28(12):987-94. doi: 10.1291/hypres.28.987.
|
| 19 |
Telmisartan inhibits CD4-positive lymphocyte migration independent of the angiotensin type 1 receptor via peroxisome proliferator-activated receptor-gamma. Hypertension. 2008 Feb;51(2):259-66. doi: 10.1161/HYPERTENSIONAHA.107.099028. Epub 2007 Dec 24.
|
| 20 |
Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor- activation in human monocytes. J Hypertens. 2012 Jan;30(1):87-96. doi: 10.1097/HJH.0b013e32834dde5f.
|
| 21 |
Comparison of the effects of telmisartan and olmesartan on home blood pressure, glucose, and lipid profiles in patients with hypertension, chronic heart failure, and metabolic syndrome. Hypertens Res. 2008 May;31(5):921-9. doi: 10.1291/hypres.31.921.
|
| 22 |
Telmisartan increases the permeability of endothelial cells through zonula occludens-1. Biol Pharm Bull. 2009 Mar;32(3):416-20. doi: 10.1248/bpb.32.416.
|
| 23 |
Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol Lett. 2018 Mar 15;285:104-112. doi: 10.1016/j.toxlet.2018.01.002. Epub 2018 Jan 3.
|
| 24 |
Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014 Sep 26;4:6437. doi: 10.1038/srep06437.
|
| 25 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 26 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 27 |
Amonafide L-malate is not a substrate for multidrug resistance proteins in secondary acute myeloid leukemia. Leukemia. 2008 Nov;22(11):2110-5.
|
| 28 |
In vitro evaluation of cytochrome P450-mediated drug interactions between cytarabine, idarubicin, itraconazole and caspofungin. Hematology. 2004 Jun;9(3):217-21.
|
| 29 |
A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J Am Soc Nephrol. 2016 Apr;27(4):1015-28. doi: 10.1681/ASN.2015010060. Epub 2015 Aug 10.
|
| 30 |
The use of biochemical markers in cardiotoxicity monitoring in patients treated for leukemia. Neoplasma. 2005;52(5):430-4.
|
| 31 |
The induction of apoptosis by daunorubicin and idarubicin in human trisomic and diabetic fibroblasts. Cell Mol Biol Lett. 2008;13(2):182-94. doi: 10.2478/s11658-007-0045-7. Epub 2008 Apr 10.
|
| 32 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
|
|
|
|
|
|