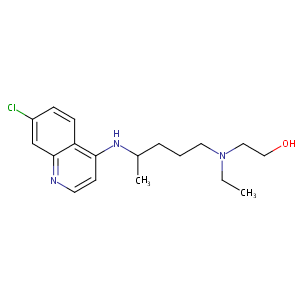
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
| DrugCom Name |
DrugCom ID |
Component Drug |
Indication |
REF |
|
Anamorelin + Hydroxychloroquine
|
DC47ZO6
|
Anamorelin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Arfolitixorin + Hydroxychloroquine
|
DCACQOC
|
Arfolitixorin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Brincidofovir + Hydroxychloroquine
|
DCG7E9X
|
Brincidofovir
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Cerivastatin + Hydroxychloroquine
|
DC3JPNJ
|
Cerivastatin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Chlorzoxazone + Hydroxychloroquine
|
DCBYB45
|
Chlorzoxazone
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Citalopram + Hydroxychloroquine
|
DCP9CR6
|
Citalopram
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Cytarabine + Hydroxychloroquine
|
DCR07DB
|
Cytarabine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Diethylcarbamazine + Hydroxychloroquine
|
DC67JV0
|
Diethylcarbamazine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Docetaxel + Hydroxychloroquine
|
DCKJSSN
|
Docetaxel
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Doxorubicin + Hydroxychloroquine
|
DC9SFS4
|
Doxorubicin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Epinephrine + Hydroxychloroquine
|
DC5EO1O
|
Epinephrine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Fomepizole + Hydroxychloroquine
|
DCD2XUN
|
Fomepizole
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Fosfomycin + Hydroxychloroquine
|
DCLDLW2
|
Fosfomycin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Aprepitant
|
DCJ81NL
|
Aprepitant
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Dantrolene
|
DC1IQIG
|
Dantrolene
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Vinblastine
|
DCMQYQH
|
Vinblastine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Tolazoline
|
DCGTMG9
|
Tolazoline
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + BIO-300
|
DC6AJ5M
|
BIO-300
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Tiagabine
|
DCXTEHU
|
Tiagabine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Diazoxide
|
DCDWWXX
|
Diazoxide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Allopurinol
|
DC8B542
|
Allopurinol
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Imatinib
|
DCCTFOL
|
Imatinib
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Bortezomib
|
DCYMPC7
|
Bortezomib
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Artemisinin
|
DC41WKW
|
Artemisinin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Trimethobenzamide
|
DCMHNH0
|
Trimethobenzamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Maprotiline
|
DCIH97K
|
Maprotiline
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Acetohexamide
|
DCF44GH
|
Acetohexamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Hyodeoxycholic acid
|
DCWABGP
|
Hyodeoxycholic acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Hydroxychloroquine + Cilostazol
|
DCSTI1U
|
Cilostazol
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Ibutilide + Hydroxychloroquine
|
DCKWPW8
|
Ibutilide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
IT-141 + Hydroxychloroquine
|
DCFBY63
|
IT-141
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Kanamycin + Hydroxychloroquine
|
DCABGRP
|
Kanamycin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Levamisole + Hydroxychloroquine
|
DCS6DUO
|
Levamisole
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Metformin + Hydroxychloroquine
|
DCX7199
|
Metformin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Methotrexate + Hydroxychloroquine
|
DCLQ05L
|
Methotrexate
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Methylene blue + Hydroxychloroquine
|
DCXRDE4
|
Methylene blue
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Metyrosine + Hydroxychloroquine
|
DCFIP0S
|
Metyrosine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Mycophenolic acid + Hydroxychloroquine
|
DCDLVQY
|
Mycophenolic acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Pinacidil + Hydroxychloroquine
|
DCFI6AQ
|
Pinacidil
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Pramipexole + Hydroxychloroquine
|
DCY7IDG
|
Pramipexole
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Pyrazinamide + Hydroxychloroquine
|
DCNCLE9
|
Pyrazinamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Repaglinide + Hydroxychloroquine
|
DCYFGRK
|
Repaglinide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Terameprocol + Hydroxychloroquine
|
DCCPHGL
|
Terameprocol
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Terazosin + Hydroxychloroquine
|
DCEQSV0
|
Terazosin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Teriflunomide + Hydroxychloroquine
|
DCONJH0
|
Teriflunomide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Tindamax + Hydroxychloroquine
|
DCW1FW9
|
Tindamax
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Triapine + Hydroxychloroquine
|
DC5292I
|
Triapine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[5] |
|
Trihexyphenidyl + Hydroxychloroquine
|
DCBCPTA
|
Trihexyphenidyl
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Trimethobenzamide + Hydroxychloroquine
|
DCNXDR6
|
Trimethobenzamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Vorinostat + Hydroxychloroquine
|
DC0EVEW
|
Vorinostat
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
|
Zaleplon + Hydroxychloroquine
|
DCSEZ6C
|
Zaleplon
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[4] |
| ------------------------------------------------------------------------------------ |
|
|
|
|