Details of the Drug Combinations
General Information of This Drug (ID: DMSYRBX)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
198904-31-3; Latazanavir; Zrivada; Reyataz; BMS-232632; BMS 232632; Atazanavir [INN:BAN]; CGP 73547; Atazanavir Base; UNII-QZU4H47A3S; CGP-73547; HSDB 7339; Reyataz (TN); ATV; QZU4H47A3S; CHEMBL1163; CHEBI:37924; (3S,8S,9S,12S)-3,12-BIS(1,1-DIMETHYLETHYL)-8-HYDROXY-4,11-DIOXO-9-(PHENYLMETHYL)-6-[[4-(2-PYRIDINYL)PHENYL]METHYL]-2,5,6,10,13-PENTAAZATETRADECANEDIOIC ACID DIMETHYL ESTER; NCGC00182552-01; AK174307; DSSTox_CID_28617; DSSTox_RID_82887; DSSTox_GSID_48691; DR7; atazanavirum; ATZ; Atazanavirum; Atazanavir (INN); Reyataz, BMS-232632, Atazanavir; Reyataz(TM) (*1:1 sulfate*); Dimethyl (3S,8S,9S,12S)-9-benzyl-3,12-di-tert-butyl-8-hydroxy-4,11-dioxo-6-[4-(2-pyridyl)benzyl]-2,5,6,10,13-pentaazatetradecanedioate; METHYL [(1S,4S,5S,10S)-4-BENZYL-1,10-DI-TERT-BUTYL-5-HYDROXY-2,9,12-TRIOXO-7-(4-PYRIDIN-2-YLBENZYL)-13-OXA-3,7,8,11-TETRAAZATETRADEC-1-YL]CARBAMATE; Methyl N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate; (2S)-N-(3-{[(2S)-2-(Methoxycarbonylamino)-3,3-dimethylbutanoylamino][(4-(2-pyridyl)phenyl)methyl]amino}(1S,2S)-2-hydroxy-1-benzylpropyl)-2-(methoxycarbonylamino)-3,3-dimethylbutanamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
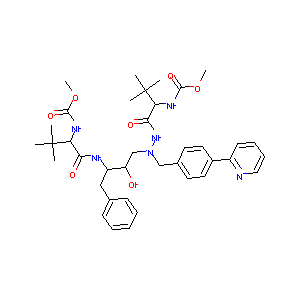 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
18 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
