Details of the Drug Combinations
General Information of This Drug (ID: DMWV3TZ)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Andozac; Eucoprost; FIT; Finasterida; Finasteridum; Finastid; Finpecia; Propecia; Propeshia; Proscar; Prostide; Cahill May Roberts Brand of Finasteride; Chibro Proscar; Frosst Iberica Brand of Finasteride; Lipha Brand of Finasteride; MSD Brand of Finasteride; MSD Chibropharm Brand of Finasteride; MK 0906; MK 906; MK906; Merck Brand 1 of Finasteride; Merck Brand 2 of Finasteride; Merck Frosst Brand 1 of Finasteride; Merck Frosst Brand 2 of Finasteride; Alternova (TN); Appecia (TN); Chibro-Proscar; Finalo (TN); Finara (TN); Finast (TN); Finasterid (TN); Finasterid IVAX (TN); Finasterida [INN-Spanish]; Finasteridum [INN-Latin]; Finax (TN); Fincar (TN); Finpecia (TN); Gefina (TN); KS-1058; MK-0906; MK-906; Merck Sharp & Dhome Brand 2 of Finasteride; Merck Sharp & Dohme Brand 1 of Finasteride; Propecia (TN); Proscar (TN); Prosteride (TN); YM-152; Finasteride (USP/INN); Finasteride [USAN:INN:BAN]; L-652,931; Proscar, Propecia, Finasteride; N-tert-Butyl-3-oxo-4-aza-5alpha-androst-1-en-17beta-carboxamide; N-tert-Butyl-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide; N-(2-methyl-2-propyl)-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide; N-(2-Methyl-2-propyl)-3-oxo-4-aza-5-alpha-androst-1-ene-17-beta-carboxamide; (1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-tert-butyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide; (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-(1,1-dimethylethyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide; (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-(tert-butyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide; (5alpha,17beta)-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide; 17beta-(N-tert-butylcarbamoyl)-4-aza-5 alpha-androst-1-en-3-one
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihyperplasia Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
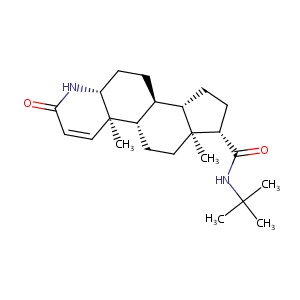 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
7 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
