| 1 |
ClinicalTrials.gov (NCT01534351) Comparison of Finasteride and Tamsulosin for Treatment of Benign Prostatic Hyperplasia (BPH) (MK-0906A-149 AM2)
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 488).
|
| 3 |
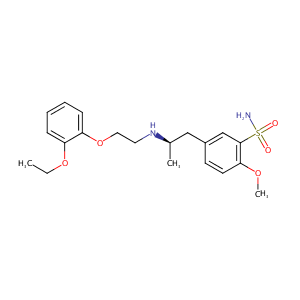
Tamsulosin FDA Label
|
| 4 |
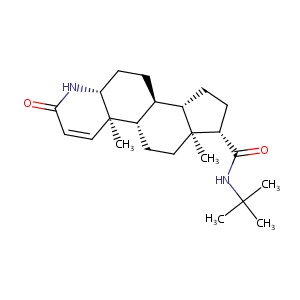
Finasteride FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6818).
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 7 |
Identification of cytochrome P450 isozymes involved in metabolism of the alpha1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998 Oct;28(10):909-22.
|
| 8 |
Cell membrane chromatography competitive binding analysis for characterization of 1A adrenoreceptor binding interactions. Anal Bioanal Chem. 2011 Jul;400(10):3625-33. doi: 10.1007/s00216-011-5026-z. Epub 2011 May 5.
|
| 9 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 10 |
The role of 5-alpha reductase inhibitors in prostate pathophysiology: Is there an additional advantage to inhibition of type 1 isoenzyme Can Urol Assoc J. 2009 Jun;3(3 Suppl 2):S109-14.
|
| 11 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 12 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 13 |
Drug Interactions Flockhart Table
|
| 14 |
Inhibition of the activity of 'basic' 5 alpha-reductase (type 1) detected in DU 145 cells and expressed in insect cells. J Steroid Biochem Mol Biol. 1994 Mar;48(4):347-52.
|
| 15 |
Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res. 2000 Dec 1;60(23):6630-40.
|
| 16 |
Effects of various pesticides on human 5alpha-reductase activity in prostate and LNCaP cells. Toxicol In Vitro. 2007 Apr;21(3):502-8.
|
| 17 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 18 |
Identification and validation of novel human pregnane X receptor activators among prescribed drugs via ligand-based virtual screening. Drug Metab Dispos. 2011 Feb;39(2):337-44. doi: 10.1124/dmd.110.035808. Epub 2010 Nov 10.
|
| 19 |
Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009 May 20;29(20):6449-60. doi: 10.1523/JNEUROSCI.0708-09.2009.
|
| 20 |
ClinicalTrials.gov (NCT01736033) Advanced Benefits of Alpha-blocker Monotherapy on Lower Urinary Tracts Symptoms(LUTS) Patients
|
|
|
|
|
|
|