Details of the Drug
General Information of Drug (ID: DMWV3TZ)
| Drug Name |
Finasteride
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Andozac; Eucoprost; FIT; Finasterida; Finasteridum; Finastid; Finpecia; Propecia; Propeshia; Proscar; Prostide; Cahill May Roberts Brand of Finasteride; Chibro Proscar; Frosst Iberica Brand of Finasteride; Lipha Brand of Finasteride; MSD Brand of Finasteride; MSD Chibropharm Brand of Finasteride; MK 0906; MK 906; MK906; Merck Brand 1 of Finasteride; Merck Brand 2 of Finasteride; Merck Frosst Brand 1 of Finasteride; Merck Frosst Brand 2 of Finasteride; Alternova (TN); Appecia (TN); Chibro-Proscar; Finalo (TN); Finara (TN); Finast (TN); Finasterid (TN); Finasterid IVAX (TN); Finasterida [INN-Spanish]; Finasteridum [INN-Latin]; Finax (TN); Fincar (TN); Finpecia (TN); Gefina (TN); KS-1058; MK-0906; MK-906; Merck Sharp & Dhome Brand 2 of Finasteride; Merck Sharp & Dohme Brand 1 of Finasteride; Propecia (TN); Proscar (TN); Prosteride (TN); YM-152; Finasteride (USP/INN); Finasteride [USAN:INN:BAN]; L-652,931; Proscar, Propecia, Finasteride; N-tert-Butyl-3-oxo-4-aza-5alpha-androst-1-en-17beta-carboxamide; N-tert-Butyl-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide; N-(2-methyl-2-propyl)-3-oxo-4-aza-5alpha-androst-1-ene-17beta-carboxamide; N-(2-Methyl-2-propyl)-3-oxo-4-aza-5-alpha-androst-1-ene-17-beta-carboxamide; (1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-tert-butyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide; (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-(1,1-dimethylethyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide; (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-(tert-butyl)-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide; (5alpha,17beta)-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide; 17beta-(N-tert-butylcarbamoyl)-4-aza-5 alpha-androst-1-en-3-one
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihyperplasia Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
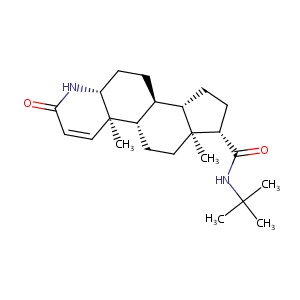 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 372.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Finasteride (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Finasteride FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6818). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | FDA Approved Drug Products: PROSCAR (finasteride) tablets | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | The role of 5-alpha reductase inhibitors in prostate pathophysiology: Is there an additional advantage to inhibition of type 1 isoenzyme Can Urol Assoc J. 2009 Jun;3(3 Suppl 2):S109-14. | ||||
| 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 9 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 10 | Drug Interactions Flockhart Table | ||||
| 11 | Inhibition of the activity of 'basic' 5 alpha-reductase (type 1) detected in DU 145 cells and expressed in insect cells. J Steroid Biochem Mol Biol. 1994 Mar;48(4):347-52. | ||||
| 12 | Effects of various pesticides on human 5alpha-reductase activity in prostate and LNCaP cells. Toxicol In Vitro. 2007 Apr;21(3):502-8. | ||||
| 13 | Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500. | ||||
| 14 | Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009 May 20;29(20):6449-60. doi: 10.1523/JNEUROSCI.0708-09.2009. | ||||
| 15 | Identification and validation of novel human pregnane X receptor activators among prescribed drugs via ligand-based virtual screening. Drug Metab Dispos. 2011 Feb;39(2):337-44. doi: 10.1124/dmd.110.035808. Epub 2010 Nov 10. | ||||
| 16 | Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res. 2000 Dec 1;60(23):6630-40. | ||||
| 17 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 20 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 21 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 22 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 23 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 24 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
