Details of the Drug Combinations
General Information of This Drug (ID: DMYCXKL)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1029712-80-8; INCB28060; INC-280; INC280; UNII-TY34L4F9OZ; 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide; INC28060; INCB-28060; INCB28060(Capmatinib); NVP-INC280; TY34L4F9OZ; Capmatinib (INCB28060); INCB 28060; 2-Fluoro-N-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-b]-[1,2,4]triazin-2-yl]benzamide; BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-; C23H17FN6O
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
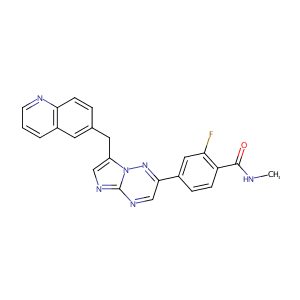 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
8 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
