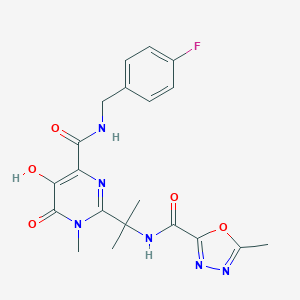
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
| DrugCom Name |
DrugCom ID |
Component Drug |
Indication |
REF |
|
Acetazolamide + Raltegravir
|
DCCU0B9
|
Acetazolamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Aprepitant + Raltegravir
|
DC0GG2V
|
Aprepitant
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Arfolitixorin + Raltegravir
|
DCYXKHL
|
Arfolitixorin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Armodafinil + Raltegravir
|
DC2DPIR
|
Armodafinil
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Auranofin + Raltegravir
|
DCTEL6A
|
Auranofin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Bepridil + Raltegravir
|
DCVO4QR
|
Bepridil
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Carglumic acid + Raltegravir
|
DCZTQOE
|
Carglumic acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Cerivastatin + Raltegravir
|
DCAAS2P
|
Cerivastatin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Chlorzoxazone + Raltegravir
|
DC3DW5K
|
Chlorzoxazone
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Clascoterone + Raltegravir
|
DCFX7ZP
|
Clascoterone
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Crotamiton + Raltegravir
|
DCMSMVV
|
Crotamiton
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Dactinomycin + Raltegravir
|
DCLO3VT
|
Dactinomycin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[3] |
|
Diazoxide + Raltegravir
|
DCAYJRX
|
Diazoxide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Dihydroergotamine + Raltegravir
|
DCJO4I8
|
Dihydroergotamine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Docetaxel + Raltegravir
|
DCV9D2P
|
Docetaxel
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
ETHISTERONE + Raltegravir
|
DC3YWDQ
|
ETHISTERONE
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Flavonoid derivative 1 + Raltegravir
|
DCXRV7G
|
Flavonoid derivative 1
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
FORMESTANE + Raltegravir
|
DCJ8GK4
|
FORMESTANE
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Gefitinib + Raltegravir
|
DC757EH
|
Gefitinib
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Idarubicin + Raltegravir
|
DC6Q1TC
|
Idarubicin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Imatinib + Raltegravir
|
DCLKJAH
|
Imatinib
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Lacosamide + Raltegravir
|
DCZD34V
|
Lacosamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Linagliptin + Raltegravir
|
DCJNOPM
|
Linagliptin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Mebendazole + Raltegravir
|
DCSL45X
|
Mebendazole
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Meclofenamic acid + Raltegravir
|
DCPBZZX
|
Meclofenamic acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Mefloquine + Raltegravir
|
DC4KDHN
|
Mefloquine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Methotrexate + Raltegravir
|
DCDU2T1
|
Methotrexate
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Mycophenolic acid + Raltegravir
|
DCK98K5
|
Mycophenolic acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Niclosamide + Raltegravir
|
DC7FJDW
|
Niclosamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Pazopanib HCl + Raltegravir
|
DC24OPM
|
Pazopanib HCl
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Pinacidil + Raltegravir
|
DCU1G8A
|
Pinacidil
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
PMID28460551-Compound-2 + Raltegravir
|
DCU9VUA
|
PMID28460551-Compound-2
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Pyrazinamide + Raltegravir
|
DCEYC9T
|
Pyrazinamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Dantrolene
|
DCVS3N7
|
Dantrolene
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Fomepizole
|
DCHQS1U
|
Fomepizole
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Triapine
|
DCJ7844
|
Triapine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + IT-141
|
DCG3RGD
|
IT-141
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + LY03004
|
DCU43WK
|
LY03004
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Fenoprofen
|
DC2Q5WD
|
Fenoprofen
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Allopurinol
|
DCBC3A7
|
Allopurinol
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Idarubicin
|
DC1N5VV
|
Idarubicin
|
Glioblastoma? (Cell Line: T98G)
|
[2] |
|
Raltegravir + Abacavir
|
DCUNDG5
|
Abacavir
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Methylene blue
|
DCZU8SN
|
Methylene blue
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Artemisinin
|
DCQ4RDS
|
Artemisinin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Leflunomide
|
DCVZM4I
|
Leflunomide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Chlorambucil
|
DCESKH1
|
Chlorambucil
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Doxorubicin
|
DCK7680
|
Doxorubicin
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Acetohydroxamic Acid
|
DCJO25J
|
Acetohydroxamic Acid
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Azatadine
|
DCKLQVU
|
Azatadine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Tetracycline
|
DCIKBTO
|
Tetracycline
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Raltegravir + Cytarabine
|
DCHT9OH
|
Cytarabine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Trimethobenzamide + Raltegravir
|
DC25S5H
|
Trimethobenzamide
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Vinblastine + Raltegravir
|
DCGBFKP
|
Vinblastine
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
|
Zileuton + Raltegravir
|
DCXBBV7
|
Zileuton
|
Chronic myelogenous leukemia (Cell Line: KBM-7)
|
[2] |
| ------------------------------------------------------------------------------------ |
|
|
|
|