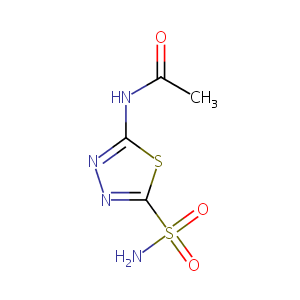
| Synonyms |
Acetadiazol; Acetamidothiadiazolesulfonamide; Acetamox; Acetazolam; Acetazolamid; Acetazolamida; Acetazolamidum; Acetazolamine; Acetazoleamide; Acetozalamide; AkZol; ApoAcetazolamide; Atenezol; Cidamex; Dazamide; Defiltran; Dehydratin; Diacarb; Diakarb; Diamox; Didoc; Diluran; Diuramid; Diuramide; Diuriwas; Diutazol; Donmox; Duiramid; Edemox; Eumicton; Fonurit; Glauconox; Glaumox; Glaupax; Glupax; HumaZolamide; Natrionex; Nephramid; Nephramide; Phonurit; Storzolamide; Vetamox; Acetazolamide Apotex Brand; Acetazolamide Chiesi Brand; Acetazolamide Dioptic Brand; Acetazolamide Grin Brand; Acetazolamide ICN Brand; Acetazolamide Jumer Brand; Acetazolamide Llorens Brand; Acetazolamide Medphano Brand; Acetazolamide Novopharm Brand; Acetazolamide Orion Brand; Acetazolamide Wassermann Brand; Ak Zol; Apo Acetazolamide; Apotex Brand of Acetazolamide; Chiesi Brand of Acetazolamide; Ciba Vision Brand of Acetazolamide; DiamoxSequels; Dioptic Brand of Acetazolamide; Grin Brand of Acetazolamide; Huma Zolamide; ICN Brand of Acetazolamide; Jumer Brand of Acetazolamide; Llorens Brand of Acetazolamide; Medphano Brand of Acetazolamide; Monosodium Salt Acetazolamide; Novopharm Brand of Acetazolamide; Orion Brand of Acetazolamide; Storz Brand of Acetazolamide Preparation; Wassermann Brand of Acetazolamide; Wyeth Brand of Acetazolamide Preparation; A 6011; Carbonic anhydrase inhibitor 6063; Acetazolamida [INN-Spanish]; Acetazolamide (AAZ); Acetazolamide, Monosodium Salt; Acetazolamidum [INN-Latin]; Ak-Zol; Apo-Acetazolamide; Carbonic Anhydrase Inhibitor No. 6063; Diamox (TN); Diureticum-holzinger; Huma-Zolamide; SK-acetazolamide; Acetazolamide Sodium, (Sterile); Acetazolamide [INN:BAN:JAN]; Acetazolamide (JP15/USP/INN); 4-Diamox
|