Details of the Drug Reposition
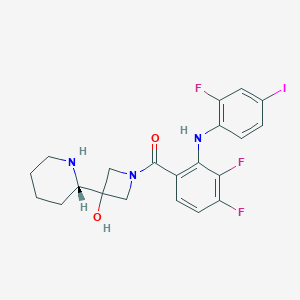
General Information of This Drug (ID: DMF9M57)
Information on Drug Reposition of This Drug
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 Phase 3 Indication(s)
|
|
||||||||||||||||||||||||||||||||||||||||
|
1 Phase 2 Indication(s)
|
|
||||||||||||||||||||||||||||||||||||||||
|
4 Phase 1 Indication(s)
|
|
||||||||||||||||||||||||||||||||||||||||
References