| Synonyms |
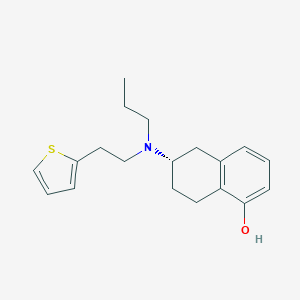
Rotigotine; 99755-59-6; Leganto; SPM 962; (S)-6-(propyl(2-(thiophen-2-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol; (6S)-rotigotine; UNII-87T4T8BO2E; (6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol; SPM-962; N 0923; CHEMBL1303; 87T4T8BO2E; 99755-59-6 (free base); (6S)-6-(Propyl(2-(2-thienyl)ethyl)amino)-5,6,7,8-tetrahydro-1-naphthalenol; NCGC00168748-01; Rotigotine CDS Patch; (6s)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthalenol; DSSTox_CID_26772; DSSTox_RID_81893; DSSTox_GSID_46772; C19H25NOS; Rotigotine CDS; (S)-5,6,7,8-Tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol; (S)-6-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-tetrahydro-naphthalen-1-ol; CAS-99755-59-6; Neupro (TN); N-0923; Rotigotine [USAN:INN:BAN]; (S)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthol; (S)-6-[Propyl[2-(thiophen-2-yl)ethyl]amino]-5,6,7,8-tetrahydronaphthalen-1-ol; SPM-936; SPM-937; PubChem16423; (-)-(S)-5,6,7,8-Tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol; GTPL941; cc-129; Rotigotine (JAN/USAN/INN); (-)-N-0437; SCHEMBL1088585; ZINC4028; DTXSID5046772; HSDB 8254; (2S)-2-[propyl-[2-(2-thienyl)ethyl]amino]tetralin-5-ol; CHEBI:135351; HMS3885D17; EX-A1164; Tox21_112627; 2696AH; BDBM50123626; MFCD00870193; s4274; AKOS016340728; Tox21_112627_1; AC-3547; CCG-267650; CS-0376; DB05271; SB19528; SS-4572; 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl (2-(2-thienyl)ethyl)amino-(6S)-; NCGC00168748-02; HY-75502; AB0088886; SW220014-1; D05768; W-5179; 572R932; A846076; Q411985; J-502471; UNII-5QTR54Z0E1 component KFQYTPMOWPVWEJ-INIZCTEOSA-N; (6S)-6-(propyl-(2-thiophen-2-ylethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol; 1-Naphthalenol, 5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-, (6S)-
|