Details of the Drug
General Information of Drug (ID: DMHEAB1)
| Drug Name |
Neupro
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rotigotine; 99755-59-6; Leganto; SPM 962; (S)-6-(propyl(2-(thiophen-2-yl)ethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol; (6S)-rotigotine; UNII-87T4T8BO2E; (6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol; SPM-962; N 0923; CHEMBL1303; 87T4T8BO2E; 99755-59-6 (free base); (6S)-6-(Propyl(2-(2-thienyl)ethyl)amino)-5,6,7,8-tetrahydro-1-naphthalenol; NCGC00168748-01; Rotigotine CDS Patch; (6s)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthalenol; DSSTox_CID_26772; DSSTox_RID_81893; DSSTox_GSID_46772; C19H25NOS; Rotigotine CDS; (S)-5,6,7,8-Tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol; (S)-6-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-tetrahydro-naphthalen-1-ol; CAS-99755-59-6; Neupro (TN); N-0923; Rotigotine [USAN:INN:BAN]; (S)-5,6,7,8-tetrahydro-6-[propyl[2-(2-thienyl)ethyl]amino]-1-naphthol; (S)-6-[Propyl[2-(thiophen-2-yl)ethyl]amino]-5,6,7,8-tetrahydronaphthalen-1-ol; SPM-936; SPM-937; PubChem16423; (-)-(S)-5,6,7,8-Tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-1-naphthol; GTPL941; cc-129; Rotigotine (JAN/USAN/INN); (-)-N-0437; SCHEMBL1088585; ZINC4028; DTXSID5046772; HSDB 8254; (2S)-2-[propyl-[2-(2-thienyl)ethyl]amino]tetralin-5-ol; CHEBI:135351; HMS3885D17; EX-A1164; Tox21_112627; 2696AH; BDBM50123626; MFCD00870193; s4274; AKOS016340728; Tox21_112627_1; AC-3547; CCG-267650; CS-0376; DB05271; SB19528; SS-4572; 1-naphthalenol, 5,6,7,8-tetrahydro-6-(propyl (2-(2-thienyl)ethyl)amino-(6S)-; NCGC00168748-02; HY-75502; AB0088886; SW220014-1; D05768; W-5179; 572R932; A846076; Q411985; J-502471; UNII-5QTR54Z0E1 component KFQYTPMOWPVWEJ-INIZCTEOSA-N; (6S)-6-(propyl-(2-thiophen-2-ylethyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol; 1-Naphthalenol, 5,6,7,8-tetrahydro-6-(propyl(2-(2-thienyl)ethyl)amino)-, (6S)-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
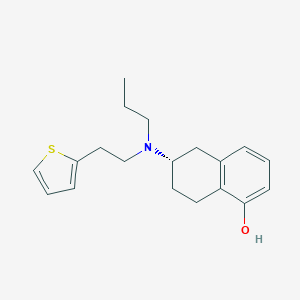 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 315.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Neupro (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | ClinicalTrials.gov (NCT01976871) Switching From Oral Dopamine Agonists to Rotigotine (SWITCH). U.S. National Institutes of Health. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01723904) A Phase 3b, Open-Label, Safety and Efficacy Study of Rotigotine as Add-On Therapy With Low Doses of Pramipexole or Ropinirole in Patients With Advanced Parkinson's Disease. U.S. National Institutes of Health. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Rotigotine Transdermal Patch: A Review in Parkinson's Disease. CNS Drugs. 2019 Jul;33(7):707-718. | ||||
| 7 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 8 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 9 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 10 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
