| Synonyms |
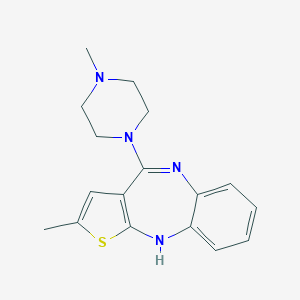
Lanzac; Midax; Olansek; Olanzapina; Olanzapinum; Symbyax; Zalasta; Zydis; Zyprexa; Eli Lilly brand of olanzapine; Lilly brand of olanzapine; Zyprexa Intramuscular; Zyprexa Velotab; Zyprexa Zydis; LY 170053; ALKS-7921; KS-1090; LY-170052; LY-170053; Olanzapine (OLA); Olanzapine [USAN:INN]; Olzapin (TN); Rexapin (TN); Zalasta (TN); Zolafren (TN); Zydis (TN); Zyprexa (TN); Olanzapine (JAN/USAN/INN); 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno(2,3-b)(1,5)benzodiazepine; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine; 2-methyl-4-(4-methyl-1-piperazinyl)-10 H-thieno[2,3-b ] [1,5]benzodiazepine; 2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3-b][1,5]benzodiazepine; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[2,3-b][1,5]benzodiazepine; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[3,2-c][1,5]benzodiazepine
|