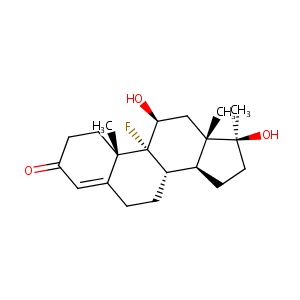
| Synonyms |
Androfluorene; Androfluorone; Androsterolo; Fluossimesterone; Fluosterone; Fluotestin; Fluoximesteron; Fluoximesterona; Fluoximesterone; Fluoximesteronum; Fluoxymesteronum; Fluoxymestrone; Flusteron; Flutestos; Halotestin; Oralsterone; Oratestin; Testoral; Ultandren; Ultandrene; Component of Halodrin; Fluossimesterone [DCIT]; Ora Testryl; U 6040; Anadroid-F; Android-f; Fluoximesterona [INN-Spanish]; Fluoxymesteronum [INN-Latin]; Halotestin (TN); Neo-Ormonal; Ora-Testryl; Fluoxymesterone [INN:BAN:JAN]; Fluoxymesterone (JP15/USP/INN); Fluoro-9-alpha dihydroxy-11-beta,17-beta methyl-17-alpha androstene-4 one-3; Fluoro-9-alpha dihydroxy-11-beta,17-beta methyl-17-alpha androstene-4 one-3 [French]; Androst-4-en-3-one, 9-fluoro-11beta,17beta-dihydroxy-17-methyl-(VAN); Androst-4-en-3-one, 9-fluoro-11beta,17beta-dihydroxy-17-methyl-(VAN) (8CI); (11beta,17beta)-9-fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one; (8S,9R,10S,11S,13S,14S,17S)-9-fluoro-11,17-dihydroxy-10,13,17-trimethyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one; 11-beta,17-beta-Dihydroxy-9-alpha-fluoro-17-alpha-methyl-4-androster-3-one; 11beta,17beta-Dihydroxy-9alpha-fluoro-17alpha-methyl-4-androsten-3-one; 11beta,17beta-Dihydroxy-9alpha-fluoro-17alpha-methyl-4-androster-3-one; 17-alpha-Methyl-9-alpha-fluoro-11-beta-hydroxytesterone; 17alpha-Methyl-9alpha-fluoro-11beta-hydroxytesterone; 9-Fluoro-11-beta,17-beta-dihydroxy-17-methylandrost-4-en-3-one; 9-Fluoro-11beta,17beta-dihydroxy-17-methylandrost-4-en-3-one; 9-alpha-Fluoro-11-beta,17-beta-dihydroxy-17-alpha-methyl-4-androstene-3-one; 9-alpha-Fluoro-11-beta-hydroxy-17-methyltestosterone; 9-alpha-Fluoro-17-alpha-methyl-11-beta,17-dihydroxy-4-androsten-3-one;9-fluoro-11beta,17beta-dihydroxy-17alpha-methylandrost-4-en-3-one; 9.alpha.-Fluoro-11.beta.,17.beta.-dihydroxy-17.alpha.-methyl-4-androstene-3-one; 9.alpha.-Fluoro-11.beta.-hydroxy-17-methyltestosterone; 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methyl-4-androstene-3-one; 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methylandrost-4-en-3-one; 9alpha-Fluoro-11beta-hydroxy-17-methyltestosterone; 9alpha-Fluoro-11beta-hydroxy-17alpha-methyltestosterone; 9alpha-Fluoro-17alpha-methyl-11beta,17-dihydroxy-4-androsten-3-one
|