Details of the Drug
General Information of Drug (ID: DMUHCF1)
| Drug Name |
Fluoxymesterone
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Androfluorene; Androfluorone; Androsterolo; Fluossimesterone; Fluosterone; Fluotestin; Fluoximesteron; Fluoximesterona; Fluoximesterone; Fluoximesteronum; Fluoxymesteronum; Fluoxymestrone; Flusteron; Flutestos; Halotestin; Oralsterone; Oratestin; Testoral; Ultandren; Ultandrene; Component of Halodrin; Fluossimesterone [DCIT]; Ora Testryl; U 6040; Anadroid-F; Android-f; Fluoximesterona [INN-Spanish]; Fluoxymesteronum [INN-Latin]; Halotestin (TN); Neo-Ormonal; Ora-Testryl; Fluoxymesterone [INN:BAN:JAN]; Fluoxymesterone (JP15/USP/INN); Fluoro-9-alpha dihydroxy-11-beta,17-beta methyl-17-alpha androstene-4 one-3; Fluoro-9-alpha dihydroxy-11-beta,17-beta methyl-17-alpha androstene-4 one-3 [French]; Androst-4-en-3-one, 9-fluoro-11beta,17beta-dihydroxy-17-methyl-(VAN); Androst-4-en-3-one, 9-fluoro-11beta,17beta-dihydroxy-17-methyl-(VAN) (8CI); (11beta,17beta)-9-fluoro-11,17-dihydroxy-17-methylandrost-4-en-3-one; (8S,9R,10S,11S,13S,14S,17S)-9-fluoro-11,17-dihydroxy-10,13,17-trimethyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one; 11-beta,17-beta-Dihydroxy-9-alpha-fluoro-17-alpha-methyl-4-androster-3-one; 11beta,17beta-Dihydroxy-9alpha-fluoro-17alpha-methyl-4-androsten-3-one; 11beta,17beta-Dihydroxy-9alpha-fluoro-17alpha-methyl-4-androster-3-one; 17-alpha-Methyl-9-alpha-fluoro-11-beta-hydroxytesterone; 17alpha-Methyl-9alpha-fluoro-11beta-hydroxytesterone; 9-Fluoro-11-beta,17-beta-dihydroxy-17-methylandrost-4-en-3-one; 9-Fluoro-11beta,17beta-dihydroxy-17-methylandrost-4-en-3-one; 9-alpha-Fluoro-11-beta,17-beta-dihydroxy-17-alpha-methyl-4-androstene-3-one; 9-alpha-Fluoro-11-beta-hydroxy-17-methyltestosterone; 9-alpha-Fluoro-17-alpha-methyl-11-beta,17-dihydroxy-4-androsten-3-one;9-fluoro-11beta,17beta-dihydroxy-17alpha-methylandrost-4-en-3-one; 9.alpha.-Fluoro-11.beta.,17.beta.-dihydroxy-17.alpha.-methyl-4-androstene-3-one; 9.alpha.-Fluoro-11.beta.-hydroxy-17-methyltestosterone; 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methyl-4-androstene-3-one; 9alpha-Fluoro-11beta,17beta-dihydroxy-17alpha-methylandrost-4-en-3-one; 9alpha-Fluoro-11beta-hydroxy-17-methyltestosterone; 9alpha-Fluoro-11beta-hydroxy-17alpha-methyltestosterone; 9alpha-Fluoro-17alpha-methyl-11beta,17-dihydroxy-4-androsten-3-one
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anabolic Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
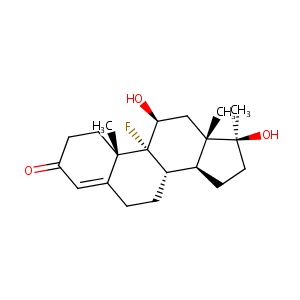 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 336.4 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Breast cancer | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C60-2C65 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Fluoxymesterone (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2861). | ||||
|---|---|---|---|---|---|
| 2 | Fluoxymesterone FDA Label | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology. 2007 Jan;148(1):363-73. | ||||
| 5 | Effect of dexamethasone on cytochrome P-450 mediated metabolism of 2-acetylaminofluorene in cultured rat hepatocytes. Biochem Pharmacol. 1987 Jan 15;36(2):237-43. | ||||
| 6 | The anabolic androgenic steroid fluoxymesterone inhibits 11beta-hydroxysteroid dehydrogenase 2-dependent glucocorticoid inactivation. Toxicol Sci. 2012 Apr;126(2):353-61. | ||||
| 7 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 8 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 9 | Asplund K, Wiholm BE, Lithner F "Glibenclamide-associated hypoglycaemia: a report on 57 cases." Diabetologia 24 (1983): 412-7. [PMID: 6411511] | ||||
| 10 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 11 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 14 | Canadian Pharmacists Association. | ||||
| 15 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 16 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
