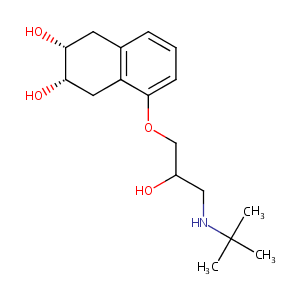
| Synonyms |
Anabet; Corgard; Corgaretic; Nadic; Nadololum; Solgol; SQ 11725; SQ11725; Anabet (TN); Corgard (TN); Corzide (TN); Nadololum [INN-Latin]; SQ-11725; Solgol (TN); Alti-Nadolol (TN); Apo-Nadol (TN); Novo-Nadolol (TN); Nadolol (JP15/USP/INN); Nadolol [USAN:BAN:INN:JAN]; (2R,3S)-5-(3-(tert-Butylamino)-2-hydroxypropoxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol; (2R,3S)-5-({3-[(1,1-dimethylethyl)amino]-2-hydroxypropyl}oxy)-1,2,3,4-tetrahydronaphthalene-2,3-diol; (2R,3S)-5-[3-(tert-butylamino)-2-hydroxypropoxy]-1,2,3,4-tetrahydronaphthalene-2,3-diol; 2,3-cis-1,2,3,4-Tetrahydro-5-((2-hydroxy-3-tert-butylamino)propoxy)-2,3-naphthalenediol; 5-(3-((1,1-Dimethylethyl)amino)-2-hydroxypropoxy)-1,2,3,4-tetrahydro-2,3-naphthalenediol
|