Details of the Drug
General Information of Drug (ID: DM0CSH8)
| Drug Name |
2-(1H-indazol-3-yl)-1H-benzo[d]imidazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3-(1H-BENZIMIDAZOL-2-YL)-1H-INDAZOLE; CHEMBL383990; 3-(1H-1,3-benzodiazol-2-yl)-2H-indazole; 2-(2H-indazol-3-yl)-1H-1,3-benzodiazole; IDZ; 1097816-83-5; Indazole Compound 2; 2c3l; AC1Q4XAS; AC1NS1P1; SCHEMBL311148; BDBM16590; 3-benzimidazol-2-yl-1h-indazole; JTKFRFMSUBOCIQ-UHFFFAOYSA-N; MolPort-016-542-829; ZINC14961821; AKOS009356321; NE25942; MCULE-6782886882; DB07959; 2-(1H-indazol-3-yl)-1H-benzimidazole; 3-(1H-benzoimidazol-2-yl)-1H-indazole; 2-(1H-Indazole-3-yl)-1H-benzoimidazole; EN300-70692
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
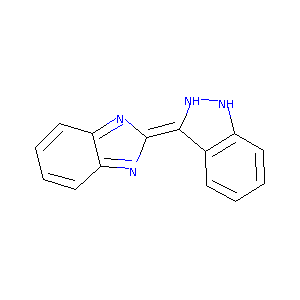 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 234.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
