Details of the Drug
General Information of Drug (ID: DM0JE6N)
| Drug Name |
Metixene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atosil; Contalyl; Dalpan; METHIXENE; Methixart; Methixen; Methyloxan; Metisene; Metixeno; Metixenum; Raunana; Tremaril;Tremarit; Tremonil; Tremoquil; Trest; Methixen [German]; Methixene hydrochloride; Metisene [DCIT]; Metixene hydrochloride; Tremaril hydrochloride; Metixene (INN); Metixene (TN); Metixene [INN:BAN]; Metixeno [INN-Spanish]; Metixenum [INN-Latin]; Methixene (*hydrochloride*); Metixene (*hydrochloride*); Tremonil (*hydrochloride*); 1-Methyl-3-(9H-thioxanthen-9-yl-methyl)piperidine; 1-Methyl-3-(9H-thioxanthen-9-ylmethyl)piperidine; 1-Methyl-3-(thioxanthen-9-ylmethyl) piperidine; 1-Methyl-3-(thioxanthen-9-ylmethyl)-1-piperidine; 1-methyl-3-(thioxanthen-9-ylmethyl)-piperidine hydrochloride; 60 SJ 1977; 60 SJ 1977 (*hydrochloride*); 9-(1'-Methylpiperidine-3'-methyl)thioxanthene
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
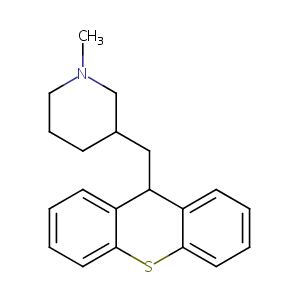 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 309.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
