Details of the Drug
General Information of Drug (ID: DM0KJDY)
| Drug Name |
LY2801653
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Merestinib; LY2801653; 1206799-15-6; LY-2801653; UNII-5OGS5K699E; N-(3-fluoro-4-((1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl)oxy)phenyl)-1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxamide; 5OGS5K699E; N-(3-Fluoro-4-{[1-Methyl-6-(1h-Pyrazol-4-Yl)-1h-Indazol-5-Yl]oxy}phenyl)-1-(4-Fluorophenyl)-6-Methyl-2-Oxo-1,2-Dihydropyridine-3-Carboxamide; C30H22F2N6O3; Merestinib [USAN]; SCHEMBL679002; LY2801653 (Merestinib); GTPL9841; CHEMBL3545307; QCR-139; DTXSID20659635; SYN1222; QHADVLVFMKEIIP-UHFFFAOYSA-N
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
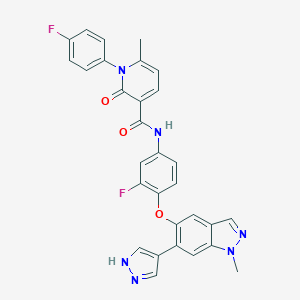 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 552.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Biliary tract cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C17 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
