Details of the Drug
General Information of Drug (ID: DM0MQ35)
| Drug Name |
Spectinomycin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Actinospectacin; Actinospectacina; Adspec; Espectinomicina; Prospec; SCM; SPCM; Spectam; Spectinomicina; Spectinomycine; Spectinomycinum; Spectogard; Stanilo; Togamycin; Trobicin; Actinospectacina [Italian]; Spectam Scour Halt; Spectinomicina [Italian]; Spectinomycin Di HCl; Spectinomycin HCl; Spectinomycin dihydrochloride; Spectinomycin hydrochloride; Spectinomycin hydrochloride anhydrous; Spectinomycin monohydrochloride; Spectinomycin sulfate; Antibiotic 2233wp; M 141; U 18409; U 18409 E; Actinospectacin, hydrochloride; Adspec (TN); Espectinomicina [INN-Spanish]; M-141; Prospec (TN); Spectinomycin (INN); Spectinomycin Dihydrochloride, Anhydrous; Spectinomycin Dihydrochloride, Pentahydrate; SpectinomycinHCl/ Sulphate; Spectinomycin Hydrochloride (anhydrous); Spectinomycin [INN:BAN]; Spectinomycine [INN-French]; Spectinomycinum [INN-Latin]; Trobicin (TN); U-18409AE; XK 43-1; Togamycin sulfate (1:1); ACTINOSPECTACIN, ESPECTINOMICINA, CHX-3101; (2R,4aR,5aR,6S,7S,8R,9S,9aR,10aS)-4a,7,9-trihydroxy-2-methyl-6,8-bis(methylamino)decahydro-4H-pyrano[2,3-b][1,4]benzodioxin-4-one; (2R-(2alpha,4abeta,5abeta,6beta,7beta,8beta,9alpha,9aalpha,10abeta))-Decahydro-4a,7,9-trihydroxy-2-methyl-6,8-bis(methylamino)-4H-pyrano(2,3-b)(1,4)benzodioxin-4-one monohydrochloride; 4H-Pyrano[2,3-b][1,4]benzodioxin-4-one, decahydro-4a,7,9-trihydroxy-2-methyl-6,8-bis(methylamino)-, [2R-(2.alpha.,4a.beta.,5a.beta.,6.beta.,7.beta.,8.beta.,9.alpha.,9a.alpha.,10a.beta.)]-, sulfate (1:1) (salt)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
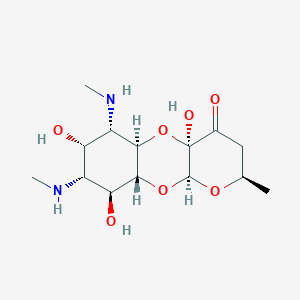 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 332.35 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Spectinomycin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
References
