Details of the Drug
General Information of Drug (ID: DM0N3L7)
| Drug Name |
Ethopropazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aethopropropazin; Athapropazine; Athopropazin; Ethapropazine; Ethopromazine; Etopropezina; Fempropazine; Fenpropazina; Isopthazine; Isotazin; Isothazine; Isothiazine; Lysivane; Parcidol; Pardidol; Parfezine; Parkin; Parkisol; Parsidan; Parsidol; Parsitan; Parsotil; Phenopropazine; Phenoprozine; Prodictazin; Prodierazine; Profenamina; Profenamine; Profenaminum; Prophenamine; Prophenaminum; Rochipel; Rocipel; Rodipal; Profenamina [Italian]; RP 3356; SC 2538; SKF 2538; W 483; Parkin (TN); Parsidan (TN); Parsidol(TN); Profenamina [INN-Spanish]; Profenamine (INN); Profenamine [INN:BAN]; Profenaminum [INN-Latin]; N,N-Diethyl-alpha-methyl-10H-phenothiazine-10-ethanamine; N,N-diethyl-1-phenothiazin-10-ylpropan-2-amine; N,N-Diethyl-1-(10H-phenothiazin-10-yl)-2-propanamine; N,N-diethyl-1-(10H-phenothiazin-10-yl)propan-2-amine; 10-(2-Diethylaminopropyl)phenothiazine; 10-[2-(Diethylamino)-1-Propyl]phenothiazine;10-[2-(Diethylamino)-2-methylethyl]phenothiazine; 10-[2-(Diethylamino)propyl]phenothiazine; 2-Diethylamino-1-propyl-N-dibenzoparathiazine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
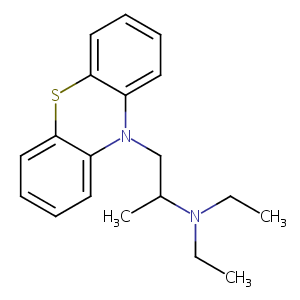 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 312.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
