Details of the Drug
General Information of Drug (ID: DM0YCPL)
| Drug Name |
Bemcentinib
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
R428; 1037624-75-1; BGB324; R-428; BGB-324; UNII-0ICW2LX8AS; (S)-1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl)-1H-1,2,4-triazole-3,5-diamine; CHEMBL3809489; SYN1131; CS-1046; HY-15150; QC-11751; R 428; W-5845; Bemcentinib [USAN]; R428 (BGB324); Bemcentinib (USAN/INN); BemcentinibR428BGB324); SCHEMBL1639904; GTPL10478; BGB 324; DTXSID70673109; 1-(6,7-dihydro-5H-benzo[2,3]cyclohepta[2,4-d]pyridazin-3-yl)-3-N-[(7S)-7-pyrrolidin-1-yl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-3-yl]-1,2,4-triazole-3,5-diamine; AMY16774; BCP21180; C30H34N8; EX-A1720; SYN-1131; BDBM50172079; NSC824183; s2841; WHO 10631; ZINC51951669; AKOS032947237; ACN-037541; DB12411; NSC-824183; SB16614; NCGC00386665-07; 1-(6,7-Dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N~3~-[(7S)-7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl]-1H-1,2,4-triazole-3,5-diamine; 1H-1,2,4-Triazole-3,5-diamine, 1-(6,7-dihydro-5H-benzo(6,7)cyclohepta(1,2-C)pyridazin-3-yl)-N3-((7S)-6,7,8,9-tetrahydro-7-(1-pyrrolidinyl)-5H-benzocyclohepten-2-yl)-; AC-28444; AS-16270; KB-80319; D11438; Q27236818; BGB324; BGB-324; BGB 324; R 428; R-428; Bemcentinib; 1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(S)-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)-1H-1,2,4-triazole-3,5-diamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
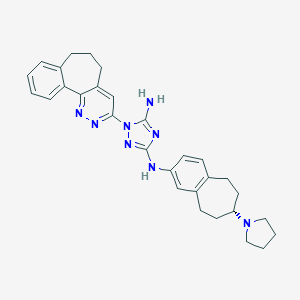 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 506.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Myelodysplastic syndrome | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A37 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
References
| 1 | ClinicalTrials.gov (NCT03824080) Evaluating the Efficacy and Safety of Bemcentinib in Patients With Myelodysplastic Syndromes. U.S. National Institutes of Health. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT03184571) Bemcentinib (BGB324) in Combination With Pembrolizumab in Patients With Advanced NSCLC. U.S. National Institutes of Health. | ||||
| 3 | ClinicalTrials.gov (NCT03184558) Bemcentinib (BGB324) in Combination With Pembrolizumab in Patients With TNBC. U.S. National Institutes of Health. | ||||
| 4 | AXL Targeting Abrogates Autophagic Flux and Induces Immunogenic Cell Death in Drug-Resistant Cancer Cells. J Thorac Oncol. 2020 Jun;15(6):973-999. | ||||
