Details of the Drug
General Information of Drug (ID: DM2PYNR)
| Drug Name |
Phenindione
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Athrombon; Bindan; Cronodione; Danedion; Danilon; Danilone; Diadilan; Dindevan; Dineval; Diophindane; Emandion; Emandione; Eridione; Fenhydren; Fenilin; Fenindion; Fenindiona; Hedulin; Hemolidione; Indema; Indion; Indon; Phenhydren; Phenillin; Phenindionum; Phenylen; Phenylin; Phenylindanedione; Phenylindione; Phenyline; Phenyllin; Pindione; Rectadione; Theradione; Thrombasal; Tromazal; Trombol; Boots Brand of Phenindione; Goldshield Brand of Phenindione; Merck Lipha Sante Brand of Phenindione; P1029; P26406_ALDRICH; Fenindiona [INN-Spanish]; Hedulin (TN); Phenindione (INN); Phenindione [INN:BAN]; Phenindionum [INN-Latin]; 2 Phenyl 1,3 indandione; 2-Fenyloindandion-1,3; 2-Fenyloindandion-1,3 [Polish]; 2-Phenyl-1,3-diketohydrindene; 2-Phenyl-1,3-indandione; 2-Phenyl-1,3-indanedione; 2-Phenyl-1H-indene-1,3(2H)-dione; 2-Phenylindan-1,3-dione; 2-Phenylindandione; 2-phenyl-1,3(2H)-Indenedione; 2-phenyl-2,3-dihydro-1H-indene-1,3-dione; 2-phenylindene-1,3-dione
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticoagulants
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
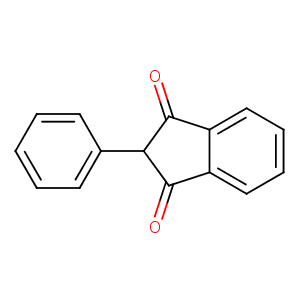 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 222.24 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
