Details of the Drug
General Information of Drug (ID: DM2WIGO)
| Drug Name |
SR9238
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
SR9238; SR 9238; 1416153-62-2; GTPL8692; SCHEMBL15773678; MolPort-042-624-584; AKOS027470306; ZINC145690538; HY-101442; CS-0021352; ethyl 5-[[[4-(3-methylsulfonylphenyl)phenyl]methyl-(2,4,6-trimethylphenyl)sulfonylamino]methyl]furan-2-carboxylate; Ethyl 5-[[[[3'-(Methylsulfonyl)[1,1'-biphenyl]-4-yl]methyl][(2,4,6-trimethylphenyl)sulfonyl]amino]methyl]-2-furancarboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
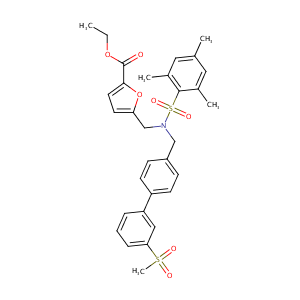 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 595.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
