Details of the Drug
General Information of Drug (ID: DM2YN37)
| Drug Name |
4-bromophenylboronic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Bromophenylboronic acid; 5467-74-3; (4-Bromophenyl)boronic acid; 4-Bromobenzeneboronic acid; 4-Bromophenylboric acid; p-Bromophenylboronic acid; p-Bromophenylboric acid; p-Bromobenzeneboronic acid; Boronic acid, (4-bromophenyl)-; Benzeneboronic acid, p-bromo-; (p-Bromophenyl)boronic acid; 4-bromo phenyl boronic acid; 4-bromophenyl boronic acid; 4-bromo-phenylboronic acid; Dihydroxy-4-bromophenylborane; EINECS 226-779-9; NSC 25407; 4-bromo phenylboronic acid; BRN 2936347; CHEMBL20866; AI3-32763; Boronic acid,B-(4-romophenyl)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
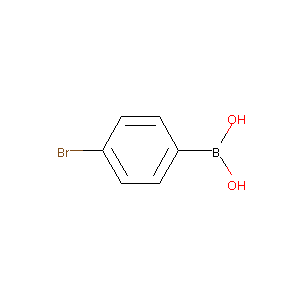 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 200.83 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
