Details of the Drug
General Information of Drug (ID: DM34GOF)
| Drug Name |
REL-1017
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dextromethadone; d-Methadone; S-Methadone; (S)-methadone; (+)-Methadone; S-(+)-Methadone; 6S-Methadone; 5653-80-5; L-(+)-Methadone; (6S)-Methadone; l-Methadone; (S)-(+)-Methadone; UNII-S95RZH8AMH; d-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; S95RZH8AMH; (S)-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; CHEMBL350719; (S)-6-(dimethylamino)-4,4-diphenylheptan-3-one; 3-14-00-00279 (Beilstein Handbook Reference); Methadone, (+)-; BRN 3213667; (+)-(S)-Methadone; (+)-(6S)-Methadone; SCHEMBL24294; (6S)-6-(dimethylamino)-4,4-diphenylheptan-3-one; CHEBI:167308; 3-HEPTANONE, 6-(DIMETHYLAMINO)-4,4-DIPHENYL-, (S)-; ZINC1530707; BDBM50223640; DB15198; SB18896; (S)-6-Dimethylamino4,4-diphenylheptan-3-on; Q15634047; (+)-(S)-6-(dimethylamino)-4,4-diphenyl-3-heptanone; 3-heptanone, 6-(dimethylamino)-4,4-diphenyl-, (6S)-; UNII-UC6VBE7V1Z component USSIQXCVUWKGNF-KRWDZBQOSA-N; UNII-UC6VBE7V1Z component USSIQXCVUWKGNF-QGZVFWFLSA-N; 3-Heptanone, 6-(dimethylamino)-4,4-diphenyl-, (6S)- (9CI); 3-Heptanone, 6-(dimethylamino)-4,4-diphenyl-, (S)- (8CI)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
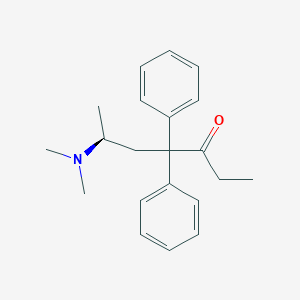 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 309.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
