| Drug Name |
PMID29671355-Compound-31
|
| Drug Type |
Small molecular drug
|
| Structure |
|
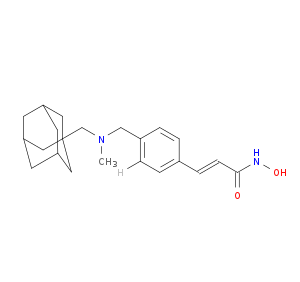 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
354.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.9 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
3 |
| Chemical Identifiers |
- Formula
- C22H30N2O2
- IUPAC Name
(E)-3-[4-[[1-adamantylmethyl(methyl)amino]methyl]phenyl]-N-hydroxyprop-2-enamide - Canonical SMILES
-
CN(CC1=CC=C(C=C1)/C=C/C(=O)NO)CC23CC4CC(C2)CC(C4)C3
- InChI
-
InChI=1S/C22H30N2O2/c1-24(14-17-4-2-16(3-5-17)6-7-21(25)23-26)15-22-11-18-8-19(12-22)10-20(9-18)13-22/h2-7,18-20,26H,8-15H2,1H3,(H,23,25)/b7-6+
- InChIKey
-
WNIDBXBLQFPAJA-VOTSOKGWSA-N
|
| Cross-matching ID |
- PubChem CID
- 90479372
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0DI9S
|
|
|
|
|
|
|
|


