Details of the Drug
General Information of Drug (ID: DM3KN7V)
| Drug Name |
Bifonazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amycor; Azolmen; Bifokey; Bifomyk; Bifon; Bifonazol; Bifonazolum; Moldina; Mycospor; Trifonazole; Bayer brand of bifonazole; Bioglan brand of bifonazole; Canesten Extra Bifonazol; Canesten brand of bifonazole; Dermapharm brand of bifonazole; Inkeysa brand of bifonazole; Juventus brand of bifonazole; Merck Lipha Sante brand of bifonazole; Bay H-4502; Bifonazol [INN-Spanish]; Bifonazolum [INN-Latin]; Canespor (TN); Mycospor (TN); Bay-h-4502; Bifonazole (JP15/USAN/INN); Bifonazole [USAN:BAN:INN:JAN]; (+-)-1-([1,1'-Biphenyl]-4-ylphenylmethyl)-1H-imidazole; (+-)-1-(p,alpha-Diphenylbenzyl)imidazole; 1-((4-Biphenylyl)phenylmethyl)-1H-imidazole; 1-(alpha-(4-Biphenylyl)benzyl)imidazole; 1-(p,alpha-Diphenylbenzyl)imidazole; 1-[4,alpha-Diphenylbenzyl]-imidazole; 1-[biphenyl-4-yl(phenyl)methyl]-1H-imidazole; 1-[phenyl-(4-phenylphenyl)methyl]imidazole
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||
| Affected Organisms |
Fungi
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
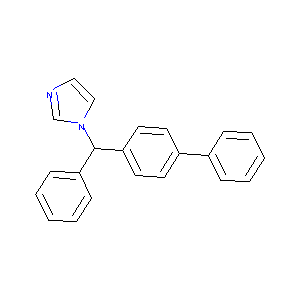 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 310.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
