Details of the Drug
General Information of Drug (ID: DM471P5)
| Drug Name |
4-hydroxybenzaldehyde
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-hydroxybenzaldehyde; p-Hydroxybenzaldehyde; 123-08-0; 4-Formylphenol; p-Formylphenol; p-Oxybenzaldehyde; Benzaldehyde, 4-hydroxy-; Parahydroxybenzaldehyde; Benzaldehyde, p-hydroxy-; 4-HYDROXY-BENZALDEHYDE; USAF M-6; 4-Hydroxy benzaldehyde; NSC 2127; p-Hydroxy-benzaldehyde; UNII-O1738X3Y38; Para-Hydroxybenzaldehyde; 4-Hydroxybenzenecarbonal; EINECS 204-599-1; BRN 0471352; CHEMBL14193; AI3-15366; PARA-HYDROXY BENZALDEHYDE; CHEBI:17597; RGHHSNMVTDWUBI-UHFFFAOYSA-N; MFCD00006939; O1738X3Y38; 4-Hydroxybenzaldehyde, 99%; Benzaldehyde, 4-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
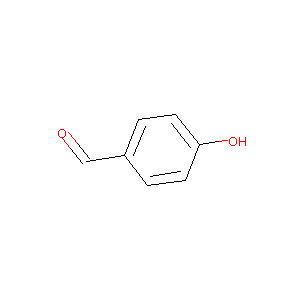 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 122.12 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
