Details of the Drug
General Information of Drug (ID: DM4URMA)
| Drug Name |
R,R-THC
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(R,R)-THC; 138090-06-9; (R,R)-cis-Diethyl tetrahydro-2,8-chrysenediol; R,R-THC; CHEMBL282489; 221368-54-3; (5R,11R)-5,11-diethyl-5,6,11,12-tetrahydrochrysene-2,8-diol; (R,R)-5,11-DIETHYL-5,6,11,12-TETRAHYDRO-2,8-CHRYSENEDIOL; (R,R)-cis-Diethyltetrahydro-2,8-chrysenediol; (R,R)-5,11-CIS-DIETHYL-5,6,11,12-TETRAHYDROCHRYSENE-2,8-DIOL; Lopac-D-8690; AC1L9K5Y; Lopac0_000463; MLS002153151; BIDD:ER0043; GTPL2822; DTXSID6040749; SCHEMBL13352540; CTK4E8744; MolPort-003-941-174; HMS3268D03; HMS3261M08; HMS2232C18; ZINC3940885; Tox21_500463
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
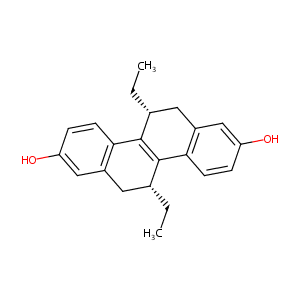 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 320.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
