Details of the Drug
General Information of Drug (ID: DM4UW65)
| Drug Name |
7-hydroxy-2-phenylchroman-4-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
7-Hydroxyflavanone; 6515-36-2; 7-Hydroxy-2-phenylchroman-4-one; 7-Hydroxyflavanone, 98%; CHEMBL97542; CHEBI:34483; SWAJPHCXKPCPQZ-UHFFFAOYSA-N; 7-hydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one; MFCD00017487; 2,3-dihydro-7-hydroxy-2-phenyl-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 2,3-dihydro-7-hydroxy-2-phenyl-; 7-hydroxy-2-phenyl-4-chromanone; 2545-13-3; 7-hydroxy-flavanone; AC1L1CGO; AC1Q6KJV; ACMC-1B5B3; Oprea1_401356; MLS001181922; SCHEMBL130266; DTXSID0022430; CTK4F5779; MolPort-002-903-717; HMS2865F07; HMS1664L06
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
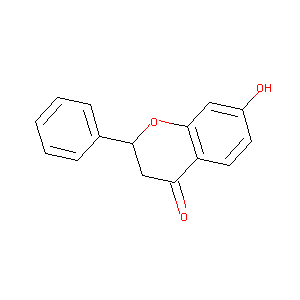 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 240.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
