Details of the Drug
General Information of Drug (ID: DM4VAOG)
| Drug Name |
Gadobenate Dimeglumine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Hydron; Multihance; Gadobenate Dimeglumine [USAN]; Gadobenic acid; Gadobenic acid dimeglumine salt; Meglumine gadobenate; Multihance Multipack; B 1903617; E-7155; Gadobenate dimeglumine (USAN); Gd-BOPTA; Meglumine gadobenate (JAN); Multihance (TN); B-19036/7; Gadolinium(3+); Gd-Bopta/dimeg; Dihydrogen ((+-)-4-carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-))gadolinate(2-), compound with 1-deoxy-1-(methylamino)-D-glucitol (1:2); (2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentol; Gadolinate(2-), (4-carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)-N5,N8,N11,O4,O5,O8,O11,O13)-, dihydrogen, comp. with 1-deoxy-1-(methylamino)-D-glucitol (1:2); 2-[2-[2-[bis(2-oxido-2-oxoethyl)amino]ethyl-(2-oxido-2-oxoethyl)amino]ethyl-(2-oxido-2-oxoethyl)amino]-3-phenylmethoxypropanoate; 2-[carboxymethyl-[2-[2-[carboxymethyl-(2-oxido-2-oxoethyl)amino]ethyl-(2-oxido-2-oxoethyl)amino]ethyl]amino]-3-phenylmethoxypropanoate; D-Glucitol, 1-deoxy-1-(methylamino)-, (4-carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)-N(sup 5),N(sup 8),N(sup 11),O(sup 4),O(sup 5),O(sup 8), O(sup 11),O(sup 13)-gadolinate(2-) (2:1); Gadolinate(2-), (4-carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)-N(sup 5),N(sup 8),N(sup 11),O(sup 4),O(sup 5),O(sup 8),O(sup 11),O(sup 13))-, dihydrogen, comp. with 1-deoxy-1-(methylamino)-D-glucitol (1:2)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Contrast Media
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
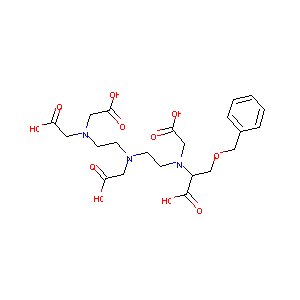 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 |
Molecular Weight | 1058.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 29 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 14 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 26 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Schizophrenia | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
