Details of the Drug
General Information of Drug (ID: DM4W8RE)
| Drug Name |
MELAGATRAN
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Melagatran; 159776-70-2; UNII-2A9QP32MD4; Melagatran [INN]; 2A9QP32MD4; CHEBI:43966; MELAGATRAN (ASTRA-ZENECA); C22H31N5O4; Melagatran (INN); N-((R)-(((2S)-2-((-p-Amidobenzyl)carbamoyl)-1-azetidinyl)carbonyl)cyclohexylmethyl)glycine; [((1R)-2-{(2S)-2-[({4-[AMINO(IMINO)METHYL]BENZYL}AMINO)CARBONYL]AZETIDINYL}-1-CYCLOHEXYL-2-OXOETHYL)AMINO]ACETIC ACID; MEL; H-319/68; N-((R)-(((2S)-2-((p-Amidinobenzyl)carbamoyl)-1-azetidinyl)carbonyl)cyclohexylmethyl)glycine
|
|||||
| ATC Code | ||||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
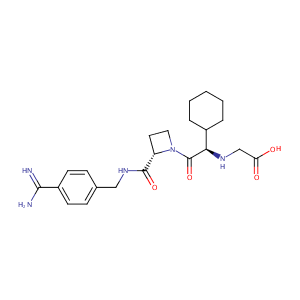 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 429.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -1 | |||||
| Rotatable Bond Count (rotbonds) | 9 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
References
